5 Steps to a 5: AP Chemistry 2024 - Moore J.T., Langley R.H. 2023
Appendixes
Pre-AP Diagnostic Exam
Summary: The AP Exam writers assume that you are bringing a certain level of knowledge to your AP Chemistry class. The AP Chemistry Exam does not test this “prior knowledge” directly, but it is still necessary to understand this material before taking the exam. For example, it is assumed that you already know that if you dissolve a strong electrolyte, such as solid NaNO3, in water, then you also know that the solution contains no NaNO3(aq) but Na+(aq) and NO3—(aq). The following sample problem is another example.
Sample Problem: Given the followed chemical equation:
![]()
what is the Ag+(aq) concentration present after mixing 10.00 mL of a 0.1000 M AgNO3 solution with 5.00 mL of a 0.1000 M K3PO4 solution? The Ksp of Ag3PO4 is 1.4 × 10—16.
The prior knowledge needed here is that you need to balance the equation before solving the problem. You should take this Pre-AP Diagnostic Exam when you first begin your study of AP Chemistry.
This Pre-AP Diagnostic Exam is for your benefit. It will test your understanding of the knowledge you should bring to your AP class. This exam will let you know where you need to spend most of your study time preparing for each topic. This does not mean you can skip the review of other topics; you should always review all topics. The exam has only multiple-choice questions and is not the mixture of multiple-choice and free-response questions that you will see on the AP Exam.
The diagnostic exam will give you an idea of where you are in terms of your preparation for your AP class and which areas you should focus on. The questions have been written to approximate the coverage of material that you will see on the AP Exam. However, there will be a few questions on content that will not be directly tested on the AP Exam; these questions refer to basic chemistry knowledge that your teacher (and the exam writers) will expect you to know and that you will need to know before taking the AP Chemistry Exam. Once you are done with the exam, check your work against the given answers, which also indicate where you can find the corresponding material in the book.

Key Ideas
• Answer questions that approximate the coverage of topics on the AP Exam.
• Check your work against the given answers.
• Determine your areas of strength and weakness.
• Highlight the topics to which you must give special attention.
Getting Started: The Pre-AP Diagnostic Exam
The following questions refer to different chapters in the book. Remember that it is not necessary to get the correct answer (though it would be nice). You will have time later to work on getting the correct answer. The problems you have trouble with will help direct your studying as you prepare for the AP Exam. If you have trouble (even if you got the correct answer), it indicates that you should spend additional time reviewing the topic. While answering these questions you may use a calculator and periodic table. For each question, circle the letter of your choice, and for each question you have difficulty with, circle the question number.
You may wish to repeat some or all questions on this exam before taking the AP Exam.
Chapter 5 Basics
1. In most of its compounds, this element exists as a monatomic anion.
(A) Fe
(B) Sr
(C) Na
(D) F
2. Which of the following groups has the species correctly listed in order of increasing radius?
(A) Cu, Cu+, Cu2+
(B) F—, Br—, I—
(C) V, V2+, V3+
(D) Li, Be, B
3. Which of the following elements has the highest electronegativity?
(A) Na
(B) B
(C) S
(D) Se
4. Which of the following represents the correct formula for perchloric acid?
(A) HClO4
(B) HClO3
(C) HClO2
(D) HClO
5. Which of the following represents the correct formula for tetraamminecopper(II) chloride?
(A) [Cu(NH3)4](ClO3)2
(B) (NH3)4Cu2Cl
(C) Am4CuCl2
(D) [Cu(NH3)4]Cl2
6. The discovery that atoms have electrons in energy levels around the nucleus is credited to which of the following?
(A) Einstein
(B) Dalton
(C) Rutherford
(D) Bohr
Chapter 6 Stoichiometry
7. 16 H+(aq) + 5 C2O42—(aq) + 2 MnO4—(aq) → 2 Mn2+(aq) + 10 CO2(g) + 8 H2O(l)
The above reaction is used in the titration of an oxalate solution. What is the concentration of the oxalate solution if it takes 42.20 mL of 0.1020 M MnO4— solution to titrate 75.00 mL of an acidified oxalate solution?
(A) 0.05741 M
(B) 0.4531 M
(C) 0.1435 M
(D) 0.2296 M
8. Chromium, Cr, forms several oxides. A sample of one of the oxides is 61.9% by mass Cr. What is the simplest formula for this oxide?
(A) CrO
(B) Cr2O3
(C) CrO2
(D) CrO3
9. 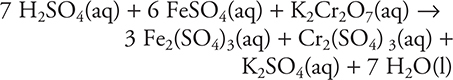
In an experiment, a chemist runs the above reaction. How many moles of Fe2(SO4)3 are produced when 1.0 mole of K2Cr2O7, 6.0 moles of FeSO4, and 6.0 moles of H2SO4 are mixed?
(A) 2.4 moles
(B) 3.0 moles
(C) 2.0 moles
(D) 3.5 moles
10. ___ H2O2(aq) → ___ H2O(l) + ___ O2(g)
After the above equation is balanced, how many moles of O2 can be produced from 1.0 mole of H2O2?
(A) 0.50 mole
(B) 1.0 mole
(C) 1.5 mole
(D) 2.0 mole
Chapter 7 Spectroscopy, Light, and Electrons
11. Which of the following groups contains only atoms that are diamagnetic in their ground state?
(A) Be, O, and Ne
(B) Ca, Ne, and Rb
(C) Mg, Kr, and Cd
(D) I, As, and Br
12. Which of the following could be the ground-state electron configuration of a transition metal ion?
(A) 1s21p62s22p2
(B) 1s22s22p63s23p64s23d104p3
(C) 1s22s22p63s23p63d9
(D) 1s22s22p4
13. Which of the following is the ground-state configuration of a noble gas?
(A) 1s22s22p63s2
(B) 1s22s22p63s23p6
(C) 1s22s22p63s23p63d5
(D) 1s22s12p6
14. Which of the following is the ground-state electron configuration of a halogen?
(A) 1s22s22p63s23p64s13d104p5
(B) 1s22s22p63s23p54s23d104p5
(C) 1s22s22p63s23p64s23d94p5
(D) 1s22s22p63s23p64s23d104p5
15. Which of the following is an impossible ground-state electron configuration?
(A) 1s22s22p63s33p64s23d104p4
(B) 1s22s22p63s23p64s23d104p5
(C) 1s22s22p63s23p64s23d104p6
(D) 1s22s22p63s23p64s23d104p3
16. Why is it not possible to determine the exact position of an electron in an atom?
(A) Pauli exclusion principle
(B) electron shielding
(C) Hund’s rule
(D) Heisenberg uncertainty principle
17. In their ground state, oxygen atoms are
paramagnetic. Why does this occur?
(A) This is due to the Pauli exclusion principle.
(B) This is due to electron shielding.
(C) This is due to Hund’s rule.
(D) This is due to the effective nuclear charge.
18. Why is it NOT possible to have a 4s3 electron configuration?
(A) Pauli exclusion principle
(B) electron shielding
(C) Hund’s rule
(D) Heisenberg uncertainty principle
19. Why does the 4s orbital fill before the 3d orbital?
(A) Pauli exclusion principle
(B) electron shielding
(C) Hund’s rule
(D) Heisenberg uncertainty principle
20. Zinc reacts with element X to form an ionic compound. If the ground-state electron configuration of X is 1s22s22p4, what is the simplest formula for this compound?
(A) ZnX2
(B) Zn2X3
(C) ZnX
(D) Zn3X2
Chapter 8 Bonding
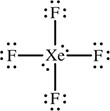
21. VSEPR predicts that an XeF4 molecule will have which of the following shapes?
(A) tetrahedral
(B) trigonal bipyramidal
(C) square planar
(D) trigonal pyramidal
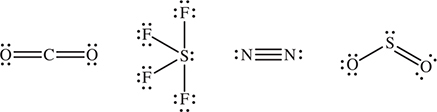
22. Which of the following does NOT have one or more π bonds?
(A) CO2
(B) SF4
(C) N2
(D) SO2
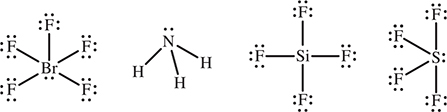
23. Which of the following molecules is nonpolar?
(A) BrF5
(B) NH3
(C) SiF4
(D) SF4
24. Which of the following substances is the only one that contains ionic, σ, and π bonds?
(A) Na2O
(B) NO3—
(C) Na2CO3
(D) PH3
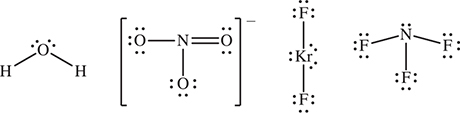
25. Which of the following species has the greatest number of unshared electron pairs around the central atom?
(A) H2O
(B) NO3—
(C) KrF2
(D) NF3
26. What is the hybridization of each carbon atom in the acetic acid CH3COOH?
(A) sp3, sp2, and sp
(B) sp3 only
(C) sp3 and sp2
(D) sp2 and sp
Chapter 9 Solids, Liquids, and Intermolecular Forces
27. Which of the following is the best description of the structure of diamond?
(A) It contains atoms held together by a delocalized electron cloud.
(B) It contains molecules held together by intermolecular attractions.
(C) It contains positive and negative ions held together by electrostatic attractions.
(D) It contains a giant molecule held together by covalent bonds.
28. Which of the following best describes the structure of Fe(s)?
(A) It contains atoms held together by a delocalized electron cloud.
(B) It contains molecules held together by intermolecular attractions.
(C) It contains positive and negative ions held together by electrostatic attractions.
(D) It contains a giant molecule held together by covalent bonds.
29. Which of the following categories best describes NH4NO3(s)?
(A) It contains atoms held together by a delocalized electron cloud.
(B) It contains molecules held together by intermolecular attractions.
(C) It contains positive and negative ions held together by electrostatic attractions.
(D) It contains a giant molecule held together by covalent bonds.
30. Which of the following is applicable to HCl(s)?
(A) It contains atoms held together by a delocalized electron cloud
(B) It contains molecules held together by intermolecular attractions.
(C) It contains positive and negative ions held together by electrostatic attractions.
(D) It contains a giant molecule held together by covalent bonds.
31. Which of the following best describes the critical point on a phase diagram?
(A) The critical point is the highest temperature and pressure where a substance can sublime.
(B) The critical point is the highest temperature and pressure where the substance may exist as discrete solid and gas phases.
(C) The critical point is the temperature and pressure where the substance exists in equilibrium as solid, liquid, and gas phases.
(D) The critical point is the highest temperature and pressure where the substance may exist as discrete liquid and gas phases.
32. Why are some substances ductile?
(A) London dispersion forces are present.
(B) Covalent bonding is present.
(C) Hydrogen bonding is present.
(D) Metallic bonding is present.
33. In the vapor phase, acetic acid, CH3COOH, molecules exist as dimers (pairs). Why do these dimers form?
(A) London dispersion forces are present.
(B) Covalent bonding is present.
(C) Hydrogen bonding is present.
(D) Metallic bonding is present.
34. Which of the following is the most likely order for boiling points of the substances?
(A) CH3CH2CH2OH < HOCH2CH2OH < CH3CH2CH2CH3
(B) CH3CH2CH2CH3 < HOCH2CH2OH < CH3CH2CH2OH
(C) CH3CH2CH2CH3 < CH3CH2CH2OH < HOCH2CH2OH
(D) CH3CH2CH2OH < CH3CH2CH2CH3 < HOCH2CH2OH
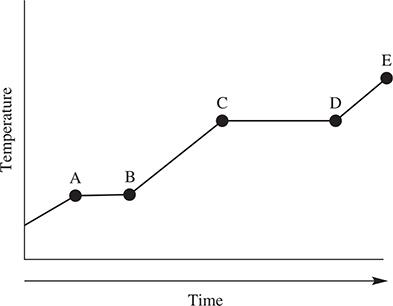
35. The above diagram represents the heating curve for a pure crystalline substance. The liquid is the only phase present between which two points?
(A) C and D
(B) A and B
(C) D and E
(D) B and C
36. For all one-component phase diagrams, choose the correct statement from the following list.
(A) The slope of the gas—liquid line may be positive or negative.
(B) The liquid—gas line terminates.
(C) The solid—liquid line has a positive slope.
(D) Sublimation occurs at temperatures higher than the triple point temperature.
Chapter 10 Gases
37. 2 Li(s) + 2 H2O(l) → 2 LiOH(aq) + H2(g)
Lithium reacts with water according to the above reaction. What volume of hydrogen gas, at standard temperature and pressure, is produced from 0.400 mole of lithium?
(A) 4.48 L
(B) 8.96 L
(C) 2.24 L
(D) 13.4 L
38. A sample of nitrogen gas is placed in a sealed container at constant pressure. The sample expands until the volume is doubled. This will also double which of the following?
(A) density
(B) number of molecules
(C) moles
(D) absolute temperature
39. A balloon contains 28 g of nitrogen gas. A second balloon contains 40 g of argon gas. Both balloons are at the same temperature and pressure. Which of the following statements is true?
(A) The number of nitrogen molecules is less than the number of argon atoms in each balloon.
(B) The density of the gas in each balloon is the same.
(C) The volume of the nitrogen balloon is less than that of the argon balloon.
(D) The average kinetic energy of the molecules/atoms in each balloon is the same.
40. The measured volume and pressure of a real gas are NOT the same as the values calculated from the ideal gas equation. Which of the following explains this discrepancy?
(A) The molecules have volume and there are attractive interactions between the molecules.
(B) The molecules have volume and the molecules have mass.
(C) There are attractive interactions between the molecules and the molecules have mass.
(D) The molecules have volume and there are variations in the absolute temperature.
41. Chromium metal reacts with gaseous HCl to produce chromium(III) chloride and hydrogen gas. What volume of dry hydrogen gas, at 1.00 atm and 25.0°C, is produced when 26.0 g of chromium is mixed with an excess of HCl?
(A) 12.2 L
(B) 16.8 L
(C) 36.7 L
(D) 18.3 L
42. A sample containing the gases methane, ethane, and propane was analyzed and found to contain 5.5 moles of methane, 3.0 moles of ethane, and 3.5 moles of propane. The mixture had a total pressure of 1.4 atm. What was the partial pressure of the ethane?
(A) 0.35 atm
(B) 0.25 atm
(C) 0.47 atm
(D) 0.30 atm
43. An ideal gas sample weighing 1.15 g at 100°C and 0.993 atm has a volume of 0.474 L. Determine the molar mass of the gas.
(A) 74.7 g mol—1
(B) 54.3 g mol—1
(C) 20.1 g mol—1
(D) 0.0134 g mol—1
44. If a sample of He effuses at a rate of 30 moles per hour at 45°C, which of the gases below will effuse at approximately 7.5 moles per hour under the same conditions?
(A) SO2
(B) NH3
(C) H2
(D) CH4
45. A sample of a gas has a volume of 10.0 L at 25°C and 760 torr pressure. What is the volume of this sample at STP?
(A) 9.16 L
(B) 10.9 L
(C) 7.21 L
(D) 10.0 L
Chapter 11 Solutions
46. A solution is prepared by dissolving 0.500 mole of KCl in 1500.0 g of water. Which of the following would be the simplest procedure to determine the molarity of the resultant solution?
(A) Measure the volume of the solution.
(B) Titrate the solution with standard silver nitrate solution.
(C) Determine the freezing point of the solution.
(D) Determine the density of the solution.
47. A chemist needs 1.0 L of a 0.50 M iodide ion, I—, solution. She has 1,000 mL of a 0.40 M KI solution. How many moles of solid MgI2 will she need to add to increase the iodide ion concentration to the desired value?
(A) 0.25 mole MgI2
(B) 0.10 mole MgI2
(C) 0.025 mole MgI2
(D) 0.050 mole MgI2
48. How many grams of H2SO4 (molar mass 98.1 g mol—1) are in 250.0 mL of a 6.00 M solution?
(A) 73.6 g H2SO4
(B) 98.1 g H2SO4
(C) 6.00 g H2SO4
(D) 147 g H2SO4
49. If a solution of chloroform, CHCl3, in carbon tetrachloride, CCl4, is treated as an ideal solution, what is the mole fraction of chloroform in the vapor over an equimolar solution of these two liquids? The vapor pressure of chloroform is 197 torr at 25°C, and the vapor pressure of carbon tetrachloride is 114 torr at this temperature.
(A) 0.387
(B) 0.500
(C) 0.631
(D) 1.63
Chapter 12 Reactions and Periodicity
50. ___ Fe(OH)2(s) + ___ H3PO4(aq) → ___ Fe3(PO4)2(s) + ___ H2O(l)
After the above chemical equation is balanced, which of the following is the lowest whole-number coefficient for water?
(A) 6
(B) 2
(C) 12
(D) 3
51. Which of the following best represents the net ionic equation for the reaction of the strong base barium hydroxide with an aqueous sodium sulfate solution to form a precipitate?
(A) Ba2+(aq) + Na2SO4(aq) → BaSO4(s) + 2 Na+(aq)
(B) 2 Ba(OH)(aq) + Na2SO4(aq) → Ba2SO4(s) + 2 NaOH(aq)
(C) Ba(OH)2(aq) + SO42—(aq) → BaSO4(s) + 2 OH—(aq)
(D) Ba2+(aq) + SO42—(aq) → BaSO4(s)
52. A student mixes 50.0 mL of a 0.10 M Ni(NO3)2 solution with 50.0-mL solution of 0.10 M NaOH. A green precipitate forms, and the concentration of the hydroxide ion becomes very low. Which of the following correctly places the concentrations of the remaining ions in order of decreasing concentration?
(A) [Ni2+] > [NO3—] > [Na+]
(B) [Ni2+] > [Na+] > [NO3—]
(C) [NO3—] > [Na+] > [Ni2+]
(D) [Na+] > [Ni2+] > [NO3—]
53. Solutions containing this ion are blue.
(A) Co2+
(B) CO32—
(C) Cu2+
(D) Ca2+
Chapter 13 Kinetics
54. Step 1: Cl2(g) ⇋ 2 Cl(g)
Step 2: Cl(g) + CH4(g) → HCl(g) + CH3(g)
Step 3: Cl(g) + CH3(g) → CH3Cl(g)
The above represents a proposed mechanism for the reaction of Cl2 with CH4. What are the overall products of the reaction?
(A) CH3Cl(g) + HCl(g)
(B) HCl(g) + CH3(g)
(C) CH3Cl(g) only
(D) CH3Cl(g) + Cl(g)
55. The decomposition reaction of NO2 to NO and O2 is second order in NO2. The rate constant of this reaction is 0.54 M —1s—1. In one experiment, the initial concentration of NO2 was 2.50 M. How long will it take the NO2 concentration to drop to 30% of the initial concentration?
(A) 0.32 s
(B) 1.7 s
(C) 0.65 s
(D) 1.8 s
56. A chemist runs a reaction (2 NO(g) + O2(g) → 2 NO2(g)) three times and collects the following data:
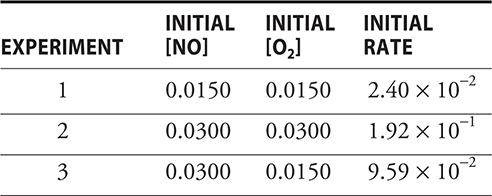
What is the rate law for this reaction?
(A) Rate = k[NO2]2[O2]2
(B) Rate = k[NO2]
(C) Rate = k[NO2]2[O2]
(D) Rate = k[O2]2
Chapter 14 Thermodynamics
57. What is the term for the energy involved when a gaseous atom in its ground state adds an electron to form a gaseous anion?
(A) ionization energy
(B) free energy
(C) anion energy
(D) electron affinity
58. Which of the following is the same for any ideal gas at a given temperature?
(A) average free energy
(B) average electron affinity
(C) average kinetic energy
(D) average ionization energy
59. Which of the following is the minimum energy required to cause a nonspontaneous reaction to occur?
(A) free energy
(B) potential energy
(C) kinetic energy
(D) spontaneous energy
60. What is the energy necessary to separate the ions from an ionic solid to infinite separation?
(A) Gibbs free energy
(B) lattice energy
(C) kinetic energy
(D) ionization energy
61. Using the following thermochemical equations, calculate the standard heat of formation for C2H2(g).
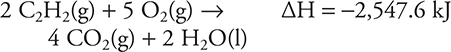
![]()
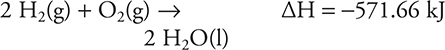
(A) +308.6 kJ
(B) +201.0 kJ
(C) 0.0 kJ
(D) -201.0 kJ
62. Choose the reaction expected to have the greatest increase in entropy.
(A) N2(g) + 2 O2(g) → 2 NO2(g)
(B) H2O(l) → H2O(g)
(C) KrF2(s) → Kr(g) + F2(g)
(D) Ca(s) + Cl2(g) → 2 CaCl2(s)
63. A certain reaction is spontaneous under standard conditions but becomes nonspontaneous at higher temperatures. What conclusions may be drawn under standard conditions?
(A) ΔH > 0, ΔS > 0, and ΔG < 0
(B) ΔH < 0, ΔS > 0, and ΔG = 0
(C) ΔH < 0, ΔS > 0, and ΔG > 0
(D) ΔH < 0, ΔS < 0, and ΔG < 0
Chapter 15 Equilibrium
64. FeS(s) + 2 H+(aq) ⇋ Fe2+(aq) + H2S(aq)
The successive acid dissociation constants for H2S are 1.0 × 10—7 (Ka1) and 1.3 × 10—13 (Ka2). The Ksp, the solubility product constant, for FeS equals 5.0 × 10—18. What is the equilibrium constant for the above reaction?
(A) 6.5 × 10—38
(B) 3.8 × 10—2
(C) 3.8 × 102
(D) 1.3 × 10—20
65. 2 NO(g) + O2(g) ⇋ 2 NO2(g) endothermic
An equilibrium mixture with each of the components is placed in a sealed container at 100°C. Which of the following changes will increase the amount of the product?
(A) lowering the temperature of the container
(B) raising the temperature of the container
(C) adding 1 mole of Ar(g) to the container
(D) removing some NO from the container
66. The Ksp for LaF3 is 2 × 10—9. What term appears in the denominator of the mass action expression (Ksp expression)?
(A) [La3+][F—]3
(B) [F—]3
(C) [LaF3]
(D) nothing
67. The Ksp for Ca3(PO4)2 is 1.2 × 10—26. What is the equilibrium reaction representing the solubility product constant (Ksp) that occurs when this substance is added to water?
(A) Ca3(PO4)2(s) ⇋ 3 Ca2+(aq) + 2 PO43—(aq)
(B) Ca3(PO4)2(s) ⇋ 3 Ca2+(aq) + 2 P5+(aq) + 8 O2—(aq)
(C) Ca3(PO4)2(s) ⇋ Ca36+(aq) + (PO4)26—(aq)
(D) Ca3(PO4)2(s) + 6 H2O(l) ⇋ 3 Ca2+(aq) + 2 PO43—(aq) + 6 H+(aq) + 6 OH—(aq)
68. A sample of copper(II) arsenate, Cu3(AsO4)2, is added to water and, at equilibrium, the copper(II) ion concentration is 1.1 × 10—7 M. What is the Ksp of copper(II) arsenate?
(A) 4.2 × 10—37
(B) 7.5 × 10—36
(C) 4.4 × 10—88
(D) 3.7 × 10—8
69. The Ksp for Cr(OH)2 is 1.0 × 10—17. What is the molar solubility of this compound in water?
(A) 3.2 × 10—9 M
(B) 1.4 × 10—6 M
(C) 1.7 × 10—6 M
(D) 2.3 × 10—49 M
Chapter 16 Acids and Bases
70. Which of the following CANNOT behave as both a Brønsted base and a Brønsted acid?
(A) HC2O4—
(B) H2PO42—
(C) HSO4—
(D) SO42—
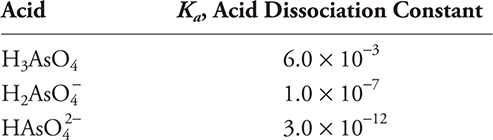
71. Using the information from the preceding table, which of the following is the best choice for preparing a pH = 7.5 buffer?
(A) K2HAsO4 + K3AsO4
(B) K2HAsO4 + KH2AsO4
(C) K3AsO4
(D) K2HAsO4
72. The pH of a 0.01-M solution of a weak monoprotic acid, HA, is 4. What is the ionization constant, Ka, for this acid?
(A) 10—4
(B) 10—6
(C) 10—8
(D) 10—2
73. Assuming all concentrations are 1 M, which of the following is the most basic solution (highest pH)?
(A) KBr (potassium bromide) and KOH
(potassium hydroxide)
(B) K2C2O4 (potassium oxalate) and KHC2O4 (potassium hydrogen oxalate)
(C) NH3 (ammonia) and HNO3 (nitric acid)
(D) (CH3)2NH (dimethylamine) and (CH3)2NH2Cl (dimethylammonium chloride)
74. Assuming all concentrations are equal, which of the following solutions has pH nearest 7?
(A) KCl (potassium chloride) and HCl (hydrochloric acid)
(B) H2C2O4 (oxalic acid) and NaOH (sodium hydroxide)
(C) NH3 (ammonia) and HNO3 (nitric acid)
(D) KOH (potassium hydroxide) and HCl (hydrochloric acid)
75. Which of the following yields a buffer with a
pH < 7 upon mixing equal volumes of 1 M
solutions?
(A) KCl (potassium chloride) and HCl
(hydrochloric acid)
(B) NH3 (ammonia) and NH4NO3 (ammonium nitrate)
(C) H2C2O4 (oxalic acid) and KHC2O4
(potassium hydrogen oxalate)
(D) KOH (potassium hydroxide) and HCl (hydrochloric acid)
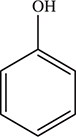
76. Which of the following is the Ka reaction for aqueous phenol, C6H5OH? The structure of phenol is given above.
(A) C6H5OH(aq) ⇋ C6H5O(aq) + H(aq)
(B) C6H5OH(aq) ⇋ C6H5O—(aq) + H+(aq)
(C) C6H5OH(aq) ⇋ C6H5(aq) + OH(aq)
(D) C6H5OH(aq) ⇋ C6H5+(aq) + OH—(aq)
77. Determine the OH—(aq) concentration in 0.01 M aniline (C6H5NH2) solution. (The Kb for aniline is Kb = 4 × 10-10.)
(A) 5 × 10—5 M
(B) 1 × 10—2 M
(C) 2 × 10—6 M
(D) 4 × 10—12 M
Chapter 17 Electrochemistry
78. (base) Bi(s) + AsO43—(aq) → AsO2—(aq) + Bi2O3(s)
What is the coefficient of OH— when the above reaction is balanced?
(A) 2
(B) 12
(C) 6
(D) 4
79. How many moles of Pt may be deposited on the cathode when 0.30 Faradays of electricity is passed through a 1.0-M solution of Pt2+?
(A) 0.60 mole
(B) 0.030 mole
(C) 0.45 mole
(D) 0.15 mole
80. Sn2+(aq) + 2 Hg2+(aq) → Sn4+(aq) + Hg22+(aq)
The reaction shown above was used in an electrolytic cell. The voltage measured for the cell was not equal to the calculated E° for the cell. Which of the following could explain this discrepancy?
(A) Both solutions were at 25°C instead of 0°C.
(B) The anode and cathode were different sizes.
(C) The anion in the anode compartment was acetate, instead of nitrate as in the cathode compartment.
(D) One or more of the ion concentrations was not 1 M.
Questions 81 and 82 refer to the following half-reaction in an electrolytic cell:
![]()
81. Choose the correct statement from the following list.
(A) The tungsten is reduced.
(B) This is the anode reaction.
(C) The oxidation state of tungsten does not change.
(D) The H+ serves as a catalyst.
82. If a current of 0.60 amperes is passed through the electrolytic cell for 0.50 h, how many grams of tungsten metal react?
(A) 0.34 g W
(B) 0.0056 g W
(C) 2.0 g W
(D) 0.68 g W
83. 4 e— + 4 H+(aq) + H2SO3(aq) → S(s) + 3 H2O(l)
If a current of 4.0 A is passed through the electrolytic cell for 0.75 h, how many grams of S will form?
(A) 3.6 g S
(B) 0.90 g S
(C) 3.2 g S
(D) 24 g
84. 2 Fe3+(aq) + Fe(s) ⇋ 3 Fe2+(aq) E° = +1.2 V
What is the ΔG° for the above reaction under standard conditions?
(A) +2.3 × 105 J
(B) -4.6 × 105 J
(C) +4.6 × 105 J
(D) -2.3 × 105 J
Chapter 18 Nuclear Chemistry
85. When ![]() decays, it emits two α particles, then two β particles, followed by an α particle. What is the resulting nucleus?
decays, it emits two α particles, then two β particles, followed by an α particle. What is the resulting nucleus?
(A) ![]()
(B) ![]()
(C) ![]()
(D) ![]()
86. Tritium, , decays by beta decay with a half-life of 12.3 years. How long will it take for a sample of tritium to decay to 12.5% of its original value?
(A) 24.6 years
(B) 49.2 years
(C) 36.9 years
(D) 12.3 years
87. The rate constant for the beta decay of carbon-14 is 1.213 × 10—4 yr—1. What is the half-life for the beta decay of carbon-14?
(A) 5,713 yr—1
(B) 1.213 × 10—4 yr—1
(C) 5,713 yr
(D) 1.213 × 10—4 yr
88. If 75% of a sample of pure decays in 30 hours, what is the half-life of ?
(A) 60 hours
(B) 30 hours
(C) 15 hours
(D) 7.5 hours
Chapter 19 Organic Chemistry
89. Alkenes are hydrocarbons with the general formula CnH2n. If a 2.80-g sample of an alkene is combusted in excess oxygen, how many moles of water will form?
(A) 0.100 mole
(B) 0.200 mole
(C) 1.50 moles
(D) 0.700 mole
90. What type of compound is shown?
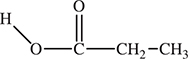
(A) an alcohol
(B) an acid
(C) a ketone
(D) an ester
Chapter 20 Experimental Investigations
Questions on this chapter are incorporated into the chapters concerning the specific experiments.
![]() Answers and Explanations
Answers and Explanations
Chapter 5 Basics
1. D—Fluorine is a nonmetal. Nonmetals tend to form anions. The others (metals) form cations.
2. B—Increasing radii is related to decreasing charges, or for going down a column (with equal charges) or moving toward the left in a period of the periodic table. This explanation will not be sufficient for the free-response portion of the test, where it is necessary to address such factors as the effective nuclear charge.
3. C—For the representative elements, the element that is nearest to F on the periodic table (except the noble gases) will have the highest electronegativity.
4. A—The others are B = chloric acid, C = chlorous acid, and D = hypochlorous acid.
5. D—You need to break the name into parts: tetraammine = (NH3)4; copper(II) = Cu2+; chloride = Cl—. Since ammonia is neutral, two Cl— are necessary to balance the Cu2+ charge.
6. D—Bohr developed what is now known as the Bohr theory of the atom to explain the emission spectra of first hydrogen (and with less success, other elements). In this theory, energy is quantized with the electrons emitting or absorbing a quantum of energy when moving from one energy level to another. Einstein’s contribution to chemistry was his explanation of the photoelectric effect. Dalton’s contribution was atomic theory. Rutherford’s contribution was that atoms have a nucleus.
Chapter 6 Stoichiometry
7. C—This is a titration problem utilizing the given equation.
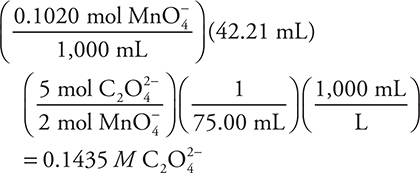
Changing M to mol/L and then substituting 1,000 mL for L simplifies the first calculation. The first two sets of parentheses determine the moles of permanganate ion. This is followed by a mole ratio to get moles of oxalate. Next, divide by the volume of the oxalate solution. Finally, convert milliliters to liters.
8. C—Either calculate the percent Cr in each oxide: (A) 76.5%; (B) 68.4%; (C) 61.9%;
(D) 52.0%, or determine the empirical formula from the percent chromium and the percent oxygen (= 100.0 - 61.9). To calculate the percent Cr for C, use the following calculation:
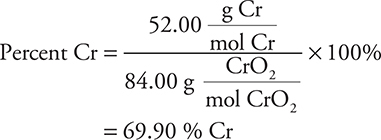
In this calculation, 84.00 g is the molar mass of CrO2. The other percentages may be calculated in a similar manner.
9. A—H2SO4 is the limiting reagent, as the amount is less than the stoichiometric ratio indicates (according to the balanced equation: 1.0 mole K2Cr2O7 and 6.0 mol FeSO4 require 7.0 moles H2SO4). The calculation is:
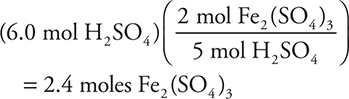
10. A—The coefficients in the balanced equation are 2, 2, and 1. Therefore,
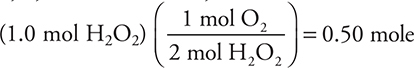
Chapter 7 Spectroscopy, Light, and Electrons
11. C—Atoms with filled shells or subshells are diamagnetic; all others are paramagnetic. All odd atomic number elements are paramagnetic in their ground states (eliminating Rb, I, As, and Br). Oxygen has a partially filled subshell, so it is also paramagnetic, which eliminates it. The paramagnetic elements eliminate answers A, B, and D.
12. C—Transition metal ions are, in general, s0 and p0 or p6 with the possibility of having one or more electrons in the d orbitals. Answer C could be Cu2+. Note that 1p (in answer A) is not a possible orbital.
13. B—The noble gases, except helium, are ns2np6. In this case, n = 3, and the gas is argon.
14. D—Halogens are ns2np5. In this case, n = 4, and the halogen is Br. None of the other configurations is a ground-state configuration.
15. A—It is not possible to have an s3 configuration.
16. D—Heisenberg’s uncertainty principle states that it is impossible to determine the exact position and momentum of an electron because any experiment to determine one of these significantly disrupts the other.
17. C—The electron configuration of a ground-state oxygen atom is 1s22s22p4. According to Hund’s rule, three of the oxygen 2p electrons will enter the 2p orbitals individually (with spins parallel). The fourth will enter one of the orbitals and pair with the electron there, which leaves two unpaired electrons. The result is that the oxygen atom has two unpaired electrons, which makes it paramagnetic.
18. A—The Pauli exclusion principle limits the number of electrons that may occupy an orbital. The two electrons present in an orbital have three quantum numbers that are the same and one electron with ms = +1/2 and one electron with ms = -1/2.
19. B—The d orbitals are less effectively shielded than the s orbitals. Due to this difference, the
s orbitals have lower energy (experience a higher effective nuclear charge, which is the result of their experiencing greater shielding).
20. C—Zn becomes Zn2+. X is O, which can become O2—. These ions yield ZnO = ZnX.
Chapter 8 Bonding
21. C—It will help if you draw the Lewis structure. The xenon has four bonding pairs and two lone pairs, which leads to a square planar molecular geometry. Answer A is four bonding pairs and no lone pairs. B has five bonding and no lone pairs. D has three bonding and one lone pair.
22. B—You will need to draw the Lewis structures. B is the only one with only single bonds. Single bonds are σ bonds. The other molecules have double or triple bonds. All double and triple bonds are a combination of σ and π bonds. It is not necessary to consider the different resonance forms.
23. C—Draw the Lewis structures and use VSEPR; only the tetrahedral SiF4 is nonpolar. The other materials form a square pyramidal, BrF5, trigonal pyramid, NH3, and irregular tetrahedral, SF4, and, therefore, are polar.
24. C—The only ionic bonds are present in the sodium compounds (eliminating B and D). The oxide ion has no internal bonding (eliminating A), but the carbonate ion has both internal σ and π bonds.
25 C—Draw the Lewis structures. The number of unshared pairs are: (A) 2; (B) 0; (C) 3; (D) 1. It is not necessary to draw resonance forms.
26. C—Draw the Lewis structure; the carbon on the left in the formula is sp3 (four single bonds), and the other is sp2 (two single and one double bonds). All carboxylic acids contain the same structure as the carbon atom on the right. Even though organic chemistry is not an AP topic, you should be able to deal with common chemicals such as acetic acid.
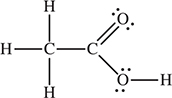
Chapter 9 Solids, Liquids, and Intermolecular Forces
27. D—Diamond is a network covalent solid with each carbon atom covalently bonded to four other carbon atoms to produce a network.
28. A—Iron is a metal; therefore, metallic bonding will be present. Answer A describes metallic bonding.
29. C—Ammonium nitrate is an ionic solid containing positive ammonium ions and negative nitrate ions.
30. B—Hydrogen chloride consists of polar molecules, which are attracted to each other through intermolecular attractions.
31. D—This is the definition of the critical point.
32. D—Ductility is a consequence of metallic bonding, as the atoms can easily move past each other without breaking any bonds. Malleability and electrical conductivity are also consequences of metallic bonding.
33. C—The structure of the dimer is illustrated in the accompanying figure. The dashed lines represent hydrogen bonds. The two strong hydrogen bonds hold the two molecules together.
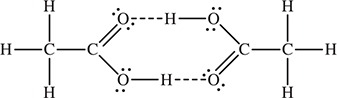
34. C—The more -OH groups, the more hydrogen bonding, and the more strongly the molecules are attracted to each other. The greater the attraction, the greater the boiling point. (Comparisons such as this require molecules of similar size.)
35. D—The solid has completely melted at point B, leaving the liquid. At point C, the liquid begins to vaporize, giving both liquid and gas.
36. B—The gas—liquid line always has a positive slope, which eliminates A. Answer B correctly describes the critical point, which is the end of the liquid—gas line. The slope of the solid—liquid line may be positive or negative, which eliminates C. Sublimation may occur at temperatures below the triple point.
Chapter 10 Gases
37.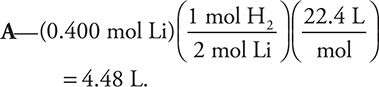
Note that the 22.4 L mol—1 only works at STP. It is also possible to use the ideal gas equation to get the correct answer. The conversion 22.4 L mol—1 is not likely to be used on the AP Exam; however, it may be used to estimate an answer.
38. D—Charles’s law applies here, as it relates volume to temperature (absolute). According to this law, volume and the absolute temperature are directly related. Thus, if either volume or temperature is doubled, the other will also double. This assumes moles and pressure remain unchanged. The container is sealed, so moles and number of molecules are constant and increasing the volume without increasing the mass will cause the density to decrease.
39. D—Each balloon contains 1 mole of gas; therefore, there are equal numbers of particles (atoms or molecules) present, and since the balloons are at the same temperature and pressure, the volumes will be the same. The average kinetic energy of a gas depends upon the temperature. Since the temperature of the two gases is the same, the average kinetic energy of the gases is the same. Density is mass over volume, and since the balloons have the same volume, the one with more mass will have the higher density.
40. A—There are two limitations with Kinetic Molecular Theory when describing an ideal gas. The volume of the molecules and the attractive interactions between the molecules are the basic differences between ideal and real gases.
41. D—It is necessary to first write the balanced chemical equation:
![]()
The quickest solution is to use the ideal gas equation (rearranged to V = nRT/P). The calculation in the square brackets [ ] is the determination of the number of moles (n).
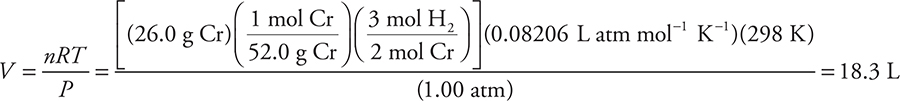
Answer A results if the chemical equation was not balanced. If you used 1 mole = 22.4 L, you got answer B, which is incorrect because this is the STP answer, and the conditions are not STP (wrong temperature).
42. A—The mole fraction of ethane times the total pressure yields the partial pressure. The mole fraction of ethane is the moles of ethane (3.0) divided by the total moles (5.5 + 3.0 + 3.5 = 12.0 moles). The calculation is 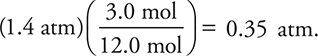 .
.
The gases are organic compounds; however, the identities of the gases are irrelevant to this calculation.
43. A—The molar mass is the mass of the gas divided by the moles of the gas. The mass is given (1.15 g), and the moles are calculated from the ideal gas equation.
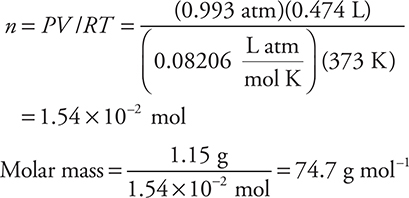
This example illustrates the importance of rounding in calculations where no calculator is available (in case you forgot to bring yours). The answers are not close together; therefore, a rough calculation will lead to the correct answer. Also, you should notice answer D is impossible for any substance. In addition, since the conditions are not STP, 22.4 L mol—1 should not appear anywhere in this calculation. If you used 22.4 L mol—1, the answer you get is 54.3 g mol—1 (answer B). If you did not change 100°C to 373K, you got answer C.
44. A—The simplest procedure is to use Graham’s law: 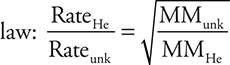 (Note: it is acceptable to invert the Rate fraction and the fraction under the radical.) Entering the rates and the molar mass of helium gives
(Note: it is acceptable to invert the Rate fraction and the fraction under the radical.) Entering the rates and the molar mass of helium gives 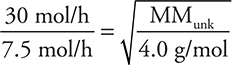 . Solving for the molar mass of the unknown gas gives MMunk = 64 g mol—1. This is reasonable since the unknown gas effuses slower than helium. The answer will be the gas with the molar mass nearest 64 g mol—1. The molar masses are SO2 = 64 g mol—1, NH3 = 17 g mol—1, H2 = 2 g mol—1 (impossible for this problem, because a lighter gas would effuse faster than He), and CH4 = 16 g mol—1.
. Solving for the molar mass of the unknown gas gives MMunk = 64 g mol—1. This is reasonable since the unknown gas effuses slower than helium. The answer will be the gas with the molar mass nearest 64 g mol—1. The molar masses are SO2 = 64 g mol—1, NH3 = 17 g mol—1, H2 = 2 g mol—1 (impossible for this problem, because a lighter gas would effuse faster than He), and CH4 = 16 g mol—1.
45. A—This is a Charles’s law problem since it will be necessary to find the new volume of the gas due to a change in temperature. (The pressure is constant since 760 torr = 1 atm = standard pressure.) The variables are V1 = 10.0 L, T1 = 25°C = 298 K,
T2 = 273 K, and V2 = ? The calculation is:
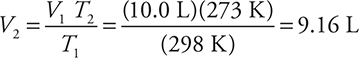
This smaller volume makes sense because there was a decrease in the temperature.
Chapter 11 Solutions
46. A—While all four methods will work, A is the simplest. Molarity is moles per liter, and the moles are already known; therefore, only the volume is necessary to complete the determination.
47. D—Moles of I— present in the desired solution: 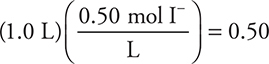 mol needed. Moles of I already present:
mol needed. Moles of I already present: 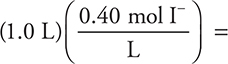 0.40 mole present. Moles MgI2 to be added:
0.40 mole present. Moles MgI2 to be added: 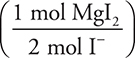 = 0.050 mol
= 0.050 mol
48. 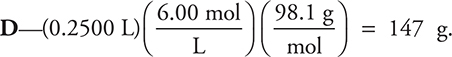 As always, estimate the answer by rounding the values.
As always, estimate the answer by rounding the values.
49. C—Equimolar gives a mole fraction of 0.500 for each. According to Raoult’s law, the vapor pressure of a component is the mole fraction of that substance times the vapor pressure of the pure substance. The total vapor pressure of the solution is the sum of the individual vapor pressures of the components: 0.500 × 197 torr +
0.500 × 114 torr = 156 torr (total vapor pressure). The mole fraction of a substance in the vapor phase is the vapor pressure of that substance divided by the total vapor pressure: chloroform = 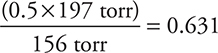 mole fraction
mole fraction
Chapter 12 Reactions and Periodicity
50. A—The balanced chemical equation is:
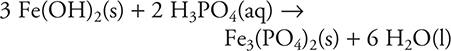
51. D—You are told that Ba(OH)2 is a strong base; therefore, it is a strong electrolyte (totally ionized in solution). You are given a solution of Na2SO4, which means it is soluble (otherwise it would not be a solution), and, for this reason, it is also a strong electrolyte. Since all reactants are strong electrolytes, the solution contains Ba2+, 2 OH—, 2 Na+, and SO42—. The only potential products from these ions are NaOH and BaSO4. Sodium hydroxide is not only a sodium compound but also a strong base, so it is expected to be soluble (strong electrolyte). By elimination, the only possible precipitate is BaSO4. The product side of the reaction will contain 2 Na+, 2 OH—, and BaSO4. The spectator ions (appearing on both sides of the reaction arrow) are 2 Na+ and 2 OH—. The final answer is what remains after canceling the spectator ions.
52. C—Both solutions have equal volumes and concentrations; therefore, the original concentrations of Ni2+, Na+, and OH— are equal, while the concentration of NO3— is double the other ions [because of the 2 subscript in Ni(NO3)2]. The hydroxide becomes low because it precipitated. The only ion available to precipitate the hydroxide is Ni2+ [the problem shows NaOH to be soluble and OH— will not combine with another anion (NO3—)]. The low OH— means that the Ni2+ will also be low. The Na+ and NO3— concentrations remain unchanged. The results are that NO3— remains the highest, Na+ is unchanged (intermediate), and Ni2+ will be the lowest.
53. C—The usual colors for these ions are: copper(II) ions, Cu2+, are blue, cobalt(II) ions, Co2+, are pink, and both carbonate ions, CO32—, and calcium ions, Ca2+, are colorless.
Chapter 13 Kinetics
54. A—Add the equations and cancel anything that appears on both sides of the reaction arrows. The overall equation is Cl2(g) + CH4(g) → CH3Cl(g) + HCl(g).
55. B—Begin with the integrated rate law for second-order reactions (this will be given to you on the AP Exam). Rearrange this equation to solve for the time, t. Finally, enter the appropriate values.
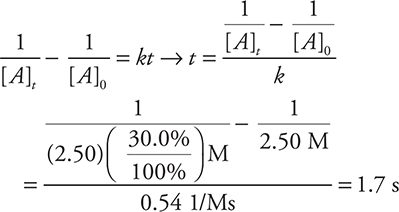
56. C—The table shows second order in nitrogen oxide (comparing experiments 1 and 3), because doubling the NO concentration quadruples (22) the rate. The reaction is first order in the oxygen (comparing experiments 2 and 3), because doubling the O2 concentration doubles (21) the rate.
Chapter 14 Thermodynamics
57. D—This is the definition of electron affinity.
58. C—This is a basic postulate of kinetic molecular theory.
59. A—This is one of the properties of free energy.
60. B—The question gives the definition of lattice energy.
61. B—This is an application of Hess’s law. The reaction for the standard heat of formation is:
![]()
(You should know how to write a chemical equation for any standard heat of formation.) According to Hess’s law, it will be necessary to manipulate the given equations so they will sum to the standard heat of formation equation. It is necessary to reverse the first equation (which reverses the sign) and divide by 2 (which cuts the ΔH to one-half its original value). The second equation needs to be doubled (which doubles ΔH). It is necessary to divide the third equation by 2 (which cuts the ΔH to one-half its original value). (You should recall that since these are thermochemical equations, it is acceptable to use fractions.) Once the equations have been manipulated, you get the three equations below; you then cancel (as shown) any species that appear on each side of the reaction arrows. Notice that O2 appears twice on the reactant side. Add all uncanceled species and compare to the standard heat of formation equation; there must be an exact match. If there is not an exact match, there is an error.
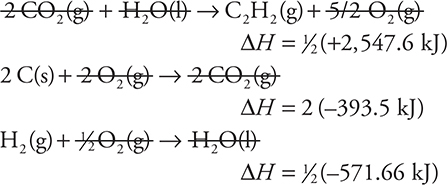
The sum to the standard heat of formation for C2H2:
![]()
As always, rounding and estimating will save time.
62. C—The equation that has the greatest increase in the number of moles of gas is the right choice. Answers A and D show a decrease in the moles of gas. Answer B has a 1-mole increase, while C has a 2-mole increase.
63. D—Spontaneous means ΔG < 0. For a reaction to become nonspontaneous at higher temperatures, (ΔG > 0) means ΔH < 0 and ΔS < 0.
Chapter 15 Equilibrium
64. C—The three equilibria involved are:
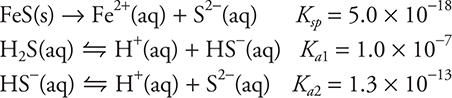
To get the equilibrium constant for the reaction in the problem, it is necessary to combine these equilibria. The two Ka equilibria need to be reversed (requiring the Ka value to be inverted). Adding the Ksp and the two reversed Ka values gives the desired equation. The resultant K is the product of the three K ’s of the equations being added.
![]()
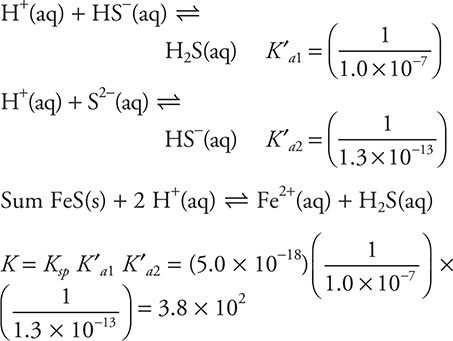
You can estimate the correct answer by using the powers of ten.
65. B—Adding Ar yields no change, as it is not part of the equilibrium. Increasing the temperature of an endothermic equilibrium will increase the amount of product. Lowering the temperature or removing a reactant (NO) will decrease the amount of product.
66. D—The equilibrium reaction is (LaF3(s) ⇌ La3+(aq) + 3 F—(aq)), and the equilibrium expression is Ksp = [La3+ ][F—]3. Solids (LaF3) do not appear in the mass action expression.
67. A—All equilibrium constant expressions for the solubility of a substance have the solid (and only the solid) on the reactant side and the individual ions on the product side (which also eliminates answer D with two substances on the reactant side). The phosphate ion is a polyatomic ion and remains a polyatomic ion in solution, which eliminates answer B. Ca36+ and (PO4)26— do not exist in solution. You should know that a phosphate ion is PO43— from your nomenclature.
68. B—The equilibrium is:
![]()
The Cu2+ concentration is 3x and the PO43- concentration is 2x and Ksp = [Cu2+]3 [PO43-]2 = (3x)3(2x)2 = 108x5. Since [Cu2+] = 3x = 1.1 × 10—7 M (given), then x = 3.7 × 10—8 M. Combining the above information: Ksp = 108x5 = 108 (3.7 × 10—8)5 = 7.5 × 10—36
69. B—The equilibrium is Cr(OH)(s) ⇌ Cr2+(aq) + 2 OH—(aq) Ksp = [Cr2+][OH—]2 = [x][2x]2 = 4x3 = 1.0 × 10—17. Solve for x = 1.4 × 10—6 M.
Chapter 16 Acids and Bases
70. D—To be a Brønsted acid, the species must have an H+ to donate, and to be a Brønsted base, the species must be able to accept an H+. The sulfate ion has no H+ to donate to be a Brønsted acid, but it can be a Brønsted base by accepting a hydrogen ion to form the hydrogen sulfate ion, HSO4—.
71. B—Start with the acid with a pKa as near 7.5 (K = 10—7.5) as possible (H2AsO4—). To go to a higher pH, add the acid (conjugate base) with the smaller Ka (higher pKa).
72. B—This is an approximation. At pH = 4, [H+] = 10—4 M = x; therefore, 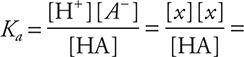
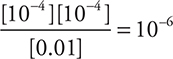
73. A—KOH is a strong base, and with equal concentrations and no acid present, it will give the highest pH.
74. D—The strong base and the strong acid will neutralize to give a neutral solution (pH = 7). This is true because the concentrations are equal. Solutions from the neutralization involving a weak acid or base will not be neutral. A is not a neutralization reaction.
75. C—Only B and C are buffers. C is acidic (pH < 7), and B is basic (pH > 7).
76. B—The desired equilibrium is a Ka; therefore, H+(aq)[or H3O+(aq)] MUST appear on the product side. The ions on the product side must have charges. In addition to the hydrogen ion, there must be an anion present for the equation to be balanced. This is a Ka, so there is only one way to write the equilibrium equation regardless of what the reacting substance is.
77. C—The general form of all Kb relationships is: Kb
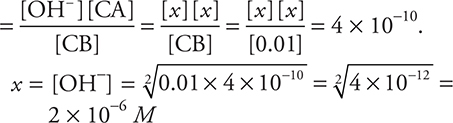
Estimate—the square root of 10-12 will be 10-6.
Chapter 17 Electrochemistry
78. C—This is a redox equation. The balanced equation is:
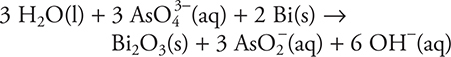
79. D—It is only necessary to know the mole ratio for the reaction [Pt2+(aq) + 2 e— → Pt(s)], which gives 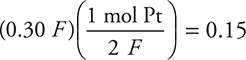 mole. Since the half-reaction has 2 e—, the reaction (and calculation) requires 2 F.
mole. Since the half-reaction has 2 e—, the reaction (and calculation) requires 2 F.
80. D—The cell must be nonstandard. This could be due to a nonstandard temperature (not 25°C) or nonstandard concentrations (not 1 M).
81. B—An oxidation is shown (tungsten loses six electrons). Oxidation only occurs at the anode.
82. A—The calculation is (check the units):
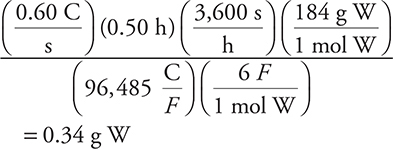
Remember: 0.60 amperes = 0.60 C/s and F = Faraday.
83. B—Recall that 4.0 amp is 4.0 C/s. The calculation would be:
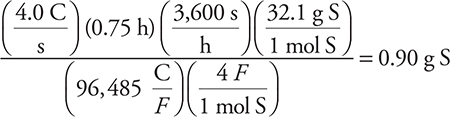
84. D—The equation is ΔG ° = —nFE °, with n = 2, F = 96,485 J V—1, and E° = +1.2 V. The value of n may be determined from either reactant, for example, Fe(s) → Fe2+(aq) + 2 e-. Entering the values into the equation gives: —(2) (96,485 J V—1) (+1.2 V) = —2.3 × 105 J.
Chapter 18 Nuclear Chemistry
85. D—The mass of an alpha particle is 4 and the mass of a beta particle is negligible. The mass number (superscript) for the missing product should be 222 - (4 + 4 + 0 + 0 + 4) = 210, which eliminates answers B and C. An alpha particle has an atomic number of 2, and, due to its charge, the “atomic number” of a beta particle is -1; therefore, the atomic number (subscript) for the missing product should be 86 - (2 + 2 - 1 - 1 + 2) = 82.
86. C—After one half-life, 50% would remain. After another half-life, this would be reduced by one-half to 25%. After three half-lives, it would be reduced by another half to leave 12.5%. Thus, three half-lives must have passed (3 × 12.3 yr) = 36.9 years. This is not a radioactivity problem (not covered on the AP Exam); it is a kinetics problem. All radioactive decay processes follow first-order kinetics. It is important not to be misled by the fact that this a kinetics problem. You could also have used the integrated rate law to determine the answer. The integrated rate law is ln [A]t - ln [A]0 = -kt.
87. C—A radioactive decay process follows first-order kinetics. The equation to determine the half-life of a first-order reaction is 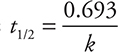 , where k is the rate constant (given). It is possible to eliminate answers A and B because the units given are not time units. Entering k into the half-life equation gives the half-life.
, where k is the rate constant (given). It is possible to eliminate answers A and B because the units given are not time units. Entering k into the half-life equation gives the half-life.
88. C—After one half-life, 50% would remain. After another half-life, this would be reduced by one-half to 25%. The total amount decayed is 75%. Thus, 30 hours must be two half-lives of 15 hours each. This is not a radioactivity problem (not covered on the AP Exam); it is a kinetics problem. All radioactive decay processes follow first-order kinetics. It is important not to be misled by the fact that this a kinetics problem.
Chapter 19 Organic Chemistry
89. B—The general combustion reaction is CnH2n(g) + 1.5 O2(g) → n CO2(g) + n H2O(g).
The general formula simplifies to CH2, which has a molar mass of 14.0 g/mol. This leads to:
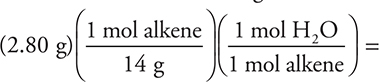
0.200 mole. While some people may consider this to be a question dealing with organic chemistry (not directly covered on the AP Exam), it is in reality a stoichiometry problem (covered on the exam). It is not unusual to see organic compounds on the AP Exam.
90. B—While organic chemistry is not an AP Exam topic, it is important to recognize some aspects of organic chemistry. The four atoms on the left side of this structure are why this compound is an acid. This collection of atoms is why acetic acid is sometimes written as CH3COOH instead of HC2H3O2. Since acetic acid commonly appears in an AP Chemistry class, it is important to know its structure.
Chapter 20 Experimental Investigations
All Chapter 20 questions have been incorporated into the chapter questions on this test concerning the specific experiments.
Scoring and Interpretation
After finishing and scoring the diagnostic exam, you need to analyze your results. During this analysis, the key is not whether you got the question correct or not, but how well you understood the question. First, you should pay attention to any area where you had difficulty (even if you got the correct answer). This should not be limited to unfamiliar material, which you will no doubt see. Determine where this material is covered in the book. Plan on spending additional time on the chapters/sections in question. There may be some material that you do not recognize simply because it was not covered in your class. Save your results for comparison whenever you retake this exam.
Note: The AP Exam assumes that you brought some information into your AP class. This “prior knowledge” is not directly tested on the exam; however, this knowledge may be necessary to fully understand some of the questions. The better you know this material, the better you will do on this exam. In addition, if you know material above and beyond what is to be tested on the exam, you will be able to do better on not only the exam but future chemistry classes.
This diagnostic exam contains no free-response questions; this is because such questions are not useful at this point. You will see many examples of free-response questions elsewhere in this book. The multiple-choice question approach is the best and quickest means of estimating your level of preparation.
If you did not do as well as you would have liked, don’t panic. There is plenty of time for you to prepare for the exam. This diagnostic exam is a guide to allow you to know which path you need to follow as you prepare for the AP Exam. This exam is not intended to be a predictor of your success.
As stated earlier, it may be useful for you to retake this exam before you start your final review. When doing so, focus on the areas where you did not improve as much as you would like.
You are about to begin your 5 Steps to a 5. Remember, this test is to help you organize your study plan and not to predict your results on the exam.
Good luck!