Cracking the AP Chemistry Exam
Part IV
Content Review for the AP Chemistry Exam
Chapter 4
Big Idea #2: Bonding and Phases
THE IDEAL GAS EQUATION
You can use the ideal gas equation to calculate any of the four variables relating to the gas, provided that you already know the other three.
The Ideal Gas Equation
PV = nRT
P = the pressure of the gas (atm)
V = the volume of the gas (L)
n = the number of moles of gas
R = the gas constant, 0.0821 L·atm/mol·K
T = the absolute temperature of the gas (K)
You can also manipulate the ideal gas equation to figure out how changes in each of its variables affect the other variables.
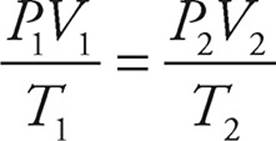
P = the pressure of the gas (atm)
V = the volume of the gas (L)
T = the absolute temperature of the gas (K)
You should be comfortable with the following simple relationships:
· If the volume is constant: As pressure increases, temperature increases; as temperature increases, pressure increases.
· If the temperature is constant: As pressure increases, volume decreases; as volume increases, pressure decreases. That’s Boyle’s law.
· If the pressure is constant: As temperature increases, volume increases; as volume increases, temperature increases. That’s Charles’s law.