Cracking the AP Chemistry Exam
Part IV
Content Review for the AP Chemistry Exam
Chapter 5
Big Idea #3: Chemical Reactions, Energy Changes, and Redox Reactions
CHAPTER 5 ANSWERS AND EXPLANATIONS
Multiple-Choice
1. B Carbonates are insoluble when paired with iron. Iron has a charge of +3 and carbonate has a charge of −2. To cancel out, both charges need to have a magnitude of 6, requiring two iron atoms and three carbonate ions. The best representation of that is choice (B).
2. D When those solutions mix, a precipitate of AgCl will form, removing those ions from the solution. The remaining ions (K+ and NO3−) do not react and remain the same as they were when they started.
3. D The precipitate that would form is PbCl2, meaning that both NO3− and Na+ are spectator ions. The precipitate would require twice as many chloride ions as lead ions. Therefore, if equal moles of both are used, the NaCl would be the limiting reagent, and almost all of the chloride ions would be present in the solid, with very few left in the solution. There would, however, still be significant Pb2+ remaining in solution, as it is in excess.
4. C Via the information given from the solubility rules, we can determine that the precipitate would be copper (I) carbonate, which has a formula of Cu2CO3. In the net ionic equation, spectator ions (in this case, K+ and Cl−) cancel out and do not appear in the final reaction.
5. C Z2+ was able to reduce to solid Z by taking electrons from metal X, so Z must have a higher reduction potential than X. Y2+ was unable to take electrons from metal X, and therefore Y must have a lower reduction potential than X.
6. A In an endothermic reaction, energy must be absorbed by the reactants for the reaction to occur. This is because the amount of energy necessary to break the bonds of the reactants is greater than the amount that is released by the formation of the bonds in the products.
7. A In (D), chromium is a pure element and has an oxidation number of 0. In (C), chromium’s oxidation number is equal to its charge of +3, and in (B) it must balance the −2 on the oxygen, so it has a charge of +2. In (A), the total charge on the ion is −2, and each oxygen is −2. Solving the following, where X is the oxidation number on chromium: X + −2(4) = −2. So, the oxidation number is +6.
8. D An activated complex occurs when the bonds of the reactants have broken, but before the bonds in the products have formed. The level must always be above those of both the reactants and products. Choice (A) is true for endothermic reactions, and choice (B) is true for exothermic reactions, but neither option is correct for all reactions.
9. D The empirical formula of boron trifluoride is BF3.
Grams = (moles)(MW)
Grams of boron = (1 mol)(10.8 g/mol) = 10.8 g
Grams of fluorine = (3 mol)(19.0 g/mol) = 57.0 g
So the mass ratio is about 57 to 11, which is about 5.3 to 1.
10. A The reaction will stop when no more precipitate can form, which will occur when either magnesium or phosphate ions run out. As there are no excess phosphate ions in solution after the reaction, the potassium phosphate must have run out first, leaving excess magnesium ions unreacted in solution.
11. D Moles = 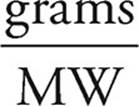
Moles of calcium hydroxide = 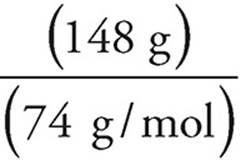 = 2 moles
= 2 moles
Every mole of Ca(OH)2 contains 2 moles of oxygen.
So there are (2)(2) = 4 moles of oxygen
Grams = (moles)(MW)
So grams of oxygen = (4 mol)(16 g/mol) = 64 grams
12. A Assume that we have 100 grams of the compound. That means that we have 31 grams of oxygen and 69 grams of chlorine.
Moles = 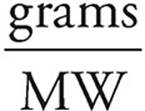
Moles of oxygen = 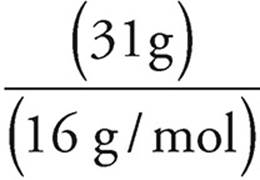 = slightly less than 2 mol
= slightly less than 2 mol
Moles of chlorine = 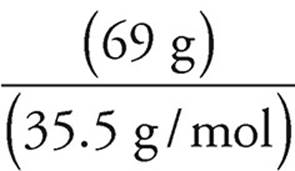 = slightly less than 2 mol
= slightly less than 2 mol
So the ratio of chlorine to oxygen is 1 to 1, and the empirical formula is ClO−.
13. C 12.25 g KClO3 × 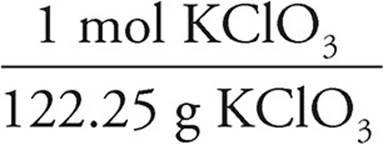 = 0.1000 mol KClO3
= 0.1000 mol KClO3
0.1000 mol KClO3 × 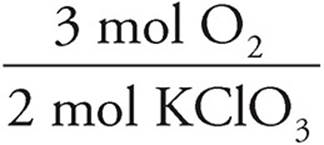 = 0.1500 mol O2
= 0.1500 mol O2
0.1500 mol O2 × 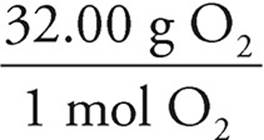 = 4.80 g O2
= 4.80 g O2
Note that even without a calculator, you are expected to be able to do simple calculations such as the ones above.
14. B 0.1500 mol O2 × 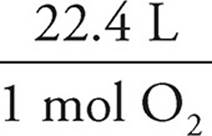 = 3.36 L O2
= 3.36 L O2
15. A Pressure is a measure of how much force the gas particles are hitting the container walls with. An increase in temperature is indicative of increased molecular velocity. If the molecules are moving faster, they will not only have more energy when they hit the container walls, but they will also hit those walls more often. Both of those factors contribute to the increased pressure.
16. D A catalyst does not change the amount of bond energy present in either the reactants or the products, and catalysts always speed up the overall reaction. Many decomposition reactions occur more quickly with a catalyst, but even without one present, the reaction can progress, albeit at a slower rate.
17. C The molecular weight of CuSO4 is 160 g/mol, so we have only 1 mole of the hydrate. The lost mass was due to water, so 1 mole of the hydrate must have contained 90 grams of H2O.
Moles = 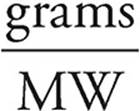
Moles of water = 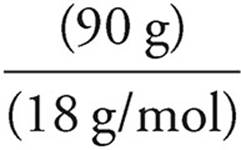 = 5 moles
= 5 moles
So if 1 mole of hydrate contains 5 moles of H2O, then the formula for the hydrate must be CuSO4 • 5 H2O.
18. B Use trial and error or backsolve.
Start with (C). If there are 3OH−, there can’t be a whole number coefficient for H2O, so (C) is wrong. You should also be able to see that the answer can’t be an odd number, so (A) is also wrong.
Try (D). If there are 4OH−, then there are 2H2O.
That leaves 2 more Os on the product side, so there must be 2CNO−.
If there are 2CNO− then there are 2CN−.
These are all whole numbers, but they are not the lowest whole numbers, so (D) is wrong.
If we divide all the coefficients by 2, we get the lowest whole number coefficients. That leaves us with 2OH−, which is choice (B).
By the way, N5− (in CN−) is oxidized to N3− (in CNO−), so there are 2e−.
19. C Moles = 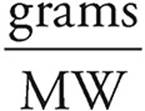
Moles of CaCO3 = 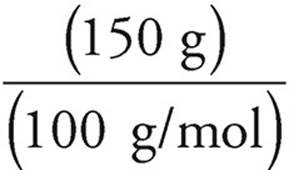 = 1.5 moles
= 1.5 moles
From the balanced equation, for every mole of CaCO3 consumed, one mole of CO2 is produced. So 1.5 moles of CO2 are produced.
At STP, volume of gas = (moles)(22.4 L)
So the volume of CO2 = (1.5)(22.4) = 34 L
20. B All of the reactants are consumed in the reaction and the temperature doesn’t change, so the pressure will change only if the number of moles of gas changes over the course of the reaction. The number of moles of gas (3 moles) doesn’t change in the balanced equation, so the pressure will remain the same (2 atm) at the end of the reaction as at the beginning.
21. D Let’s say we have 100 grams of the compound.
Moles = 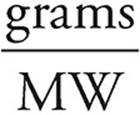
So moles of carbon = 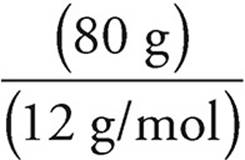 = 6.7 moles
= 6.7 moles
and moles of hydrogen = 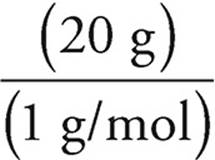 = 20 moles
= 20 moles
According to our rough calculation, there are about three times as many moles of hydrogen in the compound as there are moles of carbon, so the empirical formula is CH3.
The molar mass for the empirical formula is 15 g/mol, so we need to double the moles of each element to get a compound with a molar mass of 30 g/mol. That makes the molecular formula of the compound C2H6.
22. C The oxidation numbers of the reactants are Cr6+, O2−, I−, and H+, and the oxidation numbers of the products are Cr3+, O2−, I0, and H+.
Chromium gains electrons and is reduced, and iodine loses electrons and is oxidized; the oxidation states of oxygen and hydrogen are not changed.
23. C In a molecule, the more electronegative element takes the negative oxidation state. Hydrogen is more electronegative than calcium, so the oxidation state for Ca is +2 and the oxidation state for H is −1.
In choices (A), (B), and (D), hydrogen is the less electronegative element, and it takes the oxidation state of +1.
24. C The oxidation state of hydrogen remains +1 throughout the reaction. We have O− at the start. When water forms, oxygen has gained an electron and been reduced to O2− (GER). When O2 forms, oxygen has been oxidized to O0 through the loss of an electron (LEO). As you can see, in this process, oxygen is both oxidized and reduced.
25. A We add the reduction potential for Fe2+ (−0.4 V) to the oxidation potential for Cu (−0.3 V, the reverse of the reduction potential) to get −0.7 V.
26. D To get the reaction potential, we add the reduction potential for the reduction half-reaction to the oxidation potential for the oxidation half–reaction.
Here are the reaction potentials for choices (A)−(D).
(A) (−1.2 V) + (−0.3 V) = −1.5 V
(B) (−1.2 V) + (+0.8 V) = −0.4 V
(C) (−0.8 V) + (−0.3 V) = −1.1 V
(D) (−0.8 V) + (+1.2 V) = +0.4 V
Choice (D) is the only reaction with a positive voltage, which means that it is the only reaction listed that is favored.
27. C First let’s find out how many electrons are provided by the current.
Coulombs = (seconds)(amperes) = (600)(5.00)
Moles of electrons = 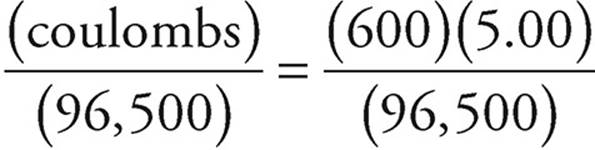 moles
moles
In AlCl3, we have Al3+, so the half-reaction for the plating of aluminum is
Al3+ + 3e− → Al(s)
So we get ![]() as many moles of Al(s) as we have moles of electrons.
as many moles of Al(s) as we have moles of electrons.
Moles of Al(s) = (moles of electrons)(![]() ) =
) = 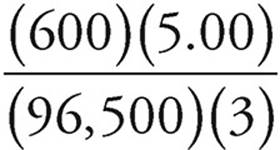 moles
moles
Now, grams = (moles)(MW)
Grams of Al(s) = 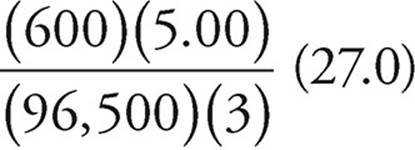 (27.0) =
(27.0) = 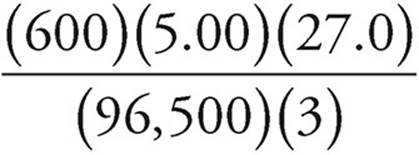 grams
grams
28. B The Pb half-cell has a greater reduction potential than the Cr half-cell. Pb2+ will be able to take electrons from the Cr(s) solution, and reduce to become Pb(s).
29. C The total net ionic equation for the cells is:
3Pb2+ + 2Cr(s) → 3Pb(s) + 2Cr3+
The Cr3+ ions are on the products side of the reaction, and the reaction would shift left, reducing the overall voltage for the cell.
30. D As the reaction progresses, Pb2+ is being reduced to create Pb(s). This decreases the amount of positive charge in the solution, which is replaced by the Na+ from the salt bridge. At the anode, excess Cr3+ is being created, which is balanced by the Cl− anions from the salt bridge.
31. A For a reaction to occur, the reduction potential of the ions in solution must exceed the reduction potential for the ions of the corresponding metal. If Cr(s) were placed in a solution containing Pb2+, the Pb2+ would take electrons from the Cr(s) and create Pb(s) and Cr3+.
Free-Response
1. (a) CaCO3 + 2HCl → CaCl2 + H2O + CO2
(b) Use the ideal gas equation to find the number of moles of CO2 produced. Remember to convert to the proper units.
n = 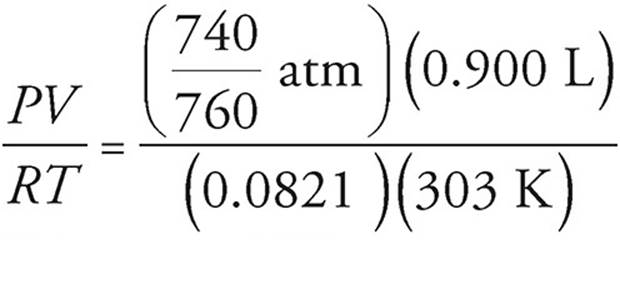 = 0.035 moles
= 0.035 moles
From the balanced equation, for every mole of CO2 produced, 1 mole of CaCO3 was consumed. So 0.035 moles of CaCO3 were consumed.
(c) We know the number of moles of CaCO3, so we can find the mass.
Grams = (moles)(MW)
Grams of CaCO3 = (0.035 mol)(100 g/mol) = 3.50 grams
Percent by mass = 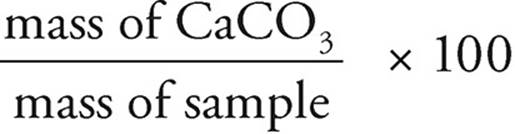 =
= 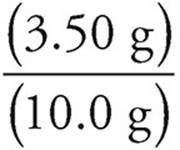 × 100 = 35%
× 100 = 35%
(d) Mass of SiO2 = 10.0 g − 3.5 g = 6.5 g
Moles = 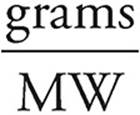
Moles of SiO2 = 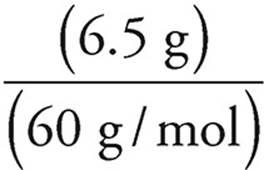 = 0.11 mol
= 0.11 mol
Molar ratio =  = 0.32
= 0.32
2. (a) All of the hydrogen in the water and all of the carbon in the carbon dioxide must have come from the hydrocarbon.
Moles of H2O = 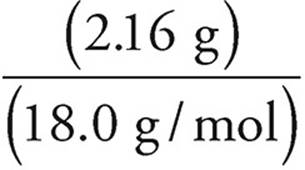 = 0.120 moles
= 0.120 moles
Every mole of water contains 2 moles of hydrogen, so there are 0.240 moles of hydrogen.
Moles of CO2 = 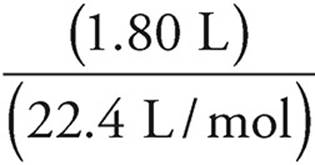 = 0.080 moles
= 0.080 moles
Every mole of CO2 contains 1 mole of carbon, so there are 0.080 moles of carbon.
There are three times as many moles of hydrogen as there are moles of carbon, so the empirical formula of the hydrocarbon is CH3.
(b) In (a), we found the number of moles of hydrogen and carbon consumed, so we can find the mass of the hydrocarbon.
Grams = (moles)(MW)
Grams of H = (0.240 mol)(1.01 g/mol) = 0.242 g
Grams of C = (0.080 mol)(12.01 g/mol) = 0.961 g
Grams of hydrocarbon = (0.242) + (0.961) = 1.203 g
(c) First let’s find the number of moles of hydrocarbon from the ideal gas law. Don’t forget to convert to the appropriate units (760 mmHg = 1 atm, 32°C = 305 K).
n = 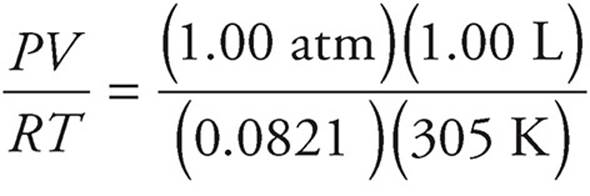 = 0.040 moles
= 0.040 moles
Now we can use the mass we found in (b) to find the molecular weight of the hydrocarbon.
MW = 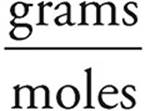 = 30.1 g/mole
= 30.1 g/mole
CH3 would have a molecular weight of 15, so we can just double the empirical formula to get the molecular formula, which is C2H6.
(d) 2C2H6 + 7O2 → 4CO2 + 6H2O
3. (a) First find the molar mass of Cu2S.
Molar mass of Cu2S = (2)(63.6) + (1)(32.1) =
159.3% by mass of Cu = 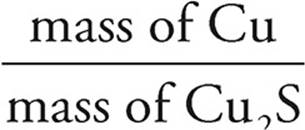 × 100 =
× 100 = 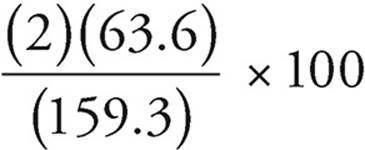 = 79.8%
= 79.8%
(b) Assume that we have 100 grams of ore #2. So we have 34.6 g of Cu, 30.5 g of Fe, and 34.9 g of S. To get the empirical formula, we need to find the number of moles of each element.
Moles =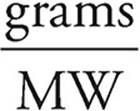
Moles of Cu = 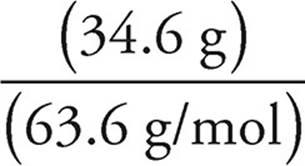 = 0.544 moles of Cu
= 0.544 moles of Cu
Moles of Fe = 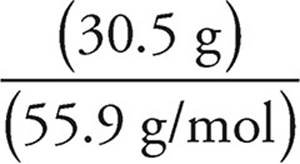 = 0.546 moles of Fe
= 0.546 moles of Fe
Moles of S = 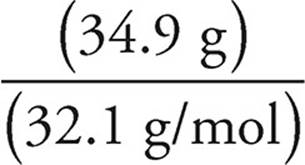 = 1.09 moles of S
= 1.09 moles of S
So the molar ratio of Cu:Fe:S is 1:1:2, and the empirical formula for ore #2 is CuFeS2.
(c) You can use the ratio of the percents by mass.
Mass of S = 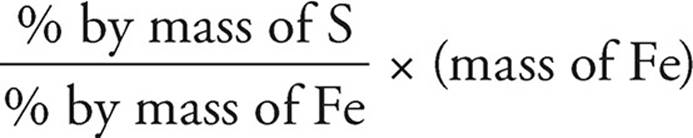 =
=
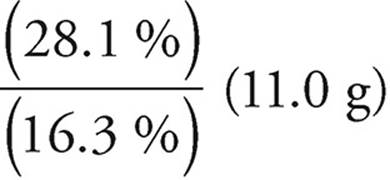 = 19.0 g
= 19.0 g
(d) First find the moles of O2 consumed.
Moles = 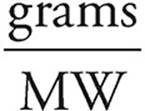
Moles of O2 = 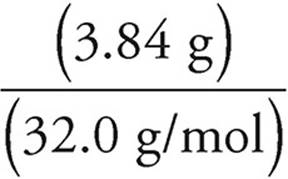 = 0.120 moles
= 0.120 moles
From the balanced equation, for every 3 moles of O2 consumed, 6 moles of Cu are produced, so the number of moles of Cu produced will be twice the number of moles of O2 consumed. So 0.240 moles of Cu are produced.
Grams = (moles)(MW)
Grams of Cu = (0.240 mol)(63.6 g/mol) = 15.3 grams
4. (a) We need to find the limiting reagent. There’s plenty of water, so it must be one of the other two reactants.
Moles = 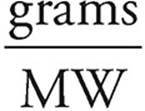
Moles of Mg = 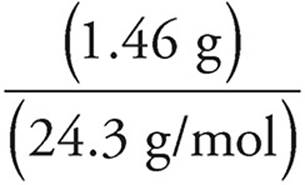 = 0.060 moles
= 0.060 moles
Moles = (molarity)(volume)
Moles of CuSO4 = (0.200 M)(0.500 L) = 0.100 moles
From the balanced equation, Mg and CuSO4 are consumed in a 1:1 ratio, so we’ll run out of Mg first. Mg is the limiting reagent, and we’ll use it to find the yield of H2.
From the balanced equation, 1 mole of H2 is produced for every 2 moles of Mg consumed, so the number of moles of H2 produced will be half the number of moles of Mg consumed.
Moles of H2 = ![]() (0.060 mol) = 0.030 moles
(0.060 mol) = 0.030 moles
(b) Mg is the limiting reagent, so some CuSO4 will remain. From the balanced equation, Mg and CuSO4 are consumed in a 1:1 ratio, so when 0.060 moles of Mg are consumed, 0.060 moles of CuSO4 are also consumed.
Moles of CuSO4 remaining = (0.100 mol) − (0.060 mol) = 0.040 moles
(c) From the balanced equation, 1 mole of Cu2O is produced for every 2 moles of Mg consumed, so the number of moles of Cu2O produced will be half the number of moles of Mg consumed.
Moles of Cu2O = ![]() (0.060 mol) = 0.030 moles
(0.060 mol) = 0.030 moles
Grams = (moles)(MW)
Grams of Cu2O = (0.030 mol)(143 g/mol) = 4.29 grams
(d) All of the Mg consumed ends up as Mg2+ ions in the solution.
Molarity = 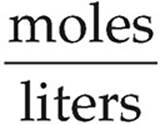
[Mg2+] = 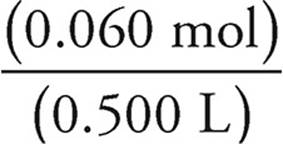 = 0.120 M
= 0.120 M
5. (a) When metal M was placed in the copper solution, a reaction occurred. Therefore, the copper must have the higher reduction potential and thus is reduced in the Cu/M cell. The half reactions are
Reduction: Cu2+ + 2e− → Cu(s)
Oxidation: M(s) → M+ + e−
The oxidation half-reaction must be multiplied by two to balance the electrons before combining the reactions to yield:
Cu2+ + 2M(s) → Cu(s) + 2M+
(b) Ered + Eox = +0.74 V
The reduction potential for Cu2+ + 2e− → Cu(s) is known:
0.34 V + Eox = +0.74 V
Eox = +0.40 V
The reduction potential for metal M is the opposite of its oxidation potential.
Ered = −0.40 V
(c) Reduction occurs at the cathode, so copper is the cathode. Oxidation occurs at the anode, so M is the anode.
(d) 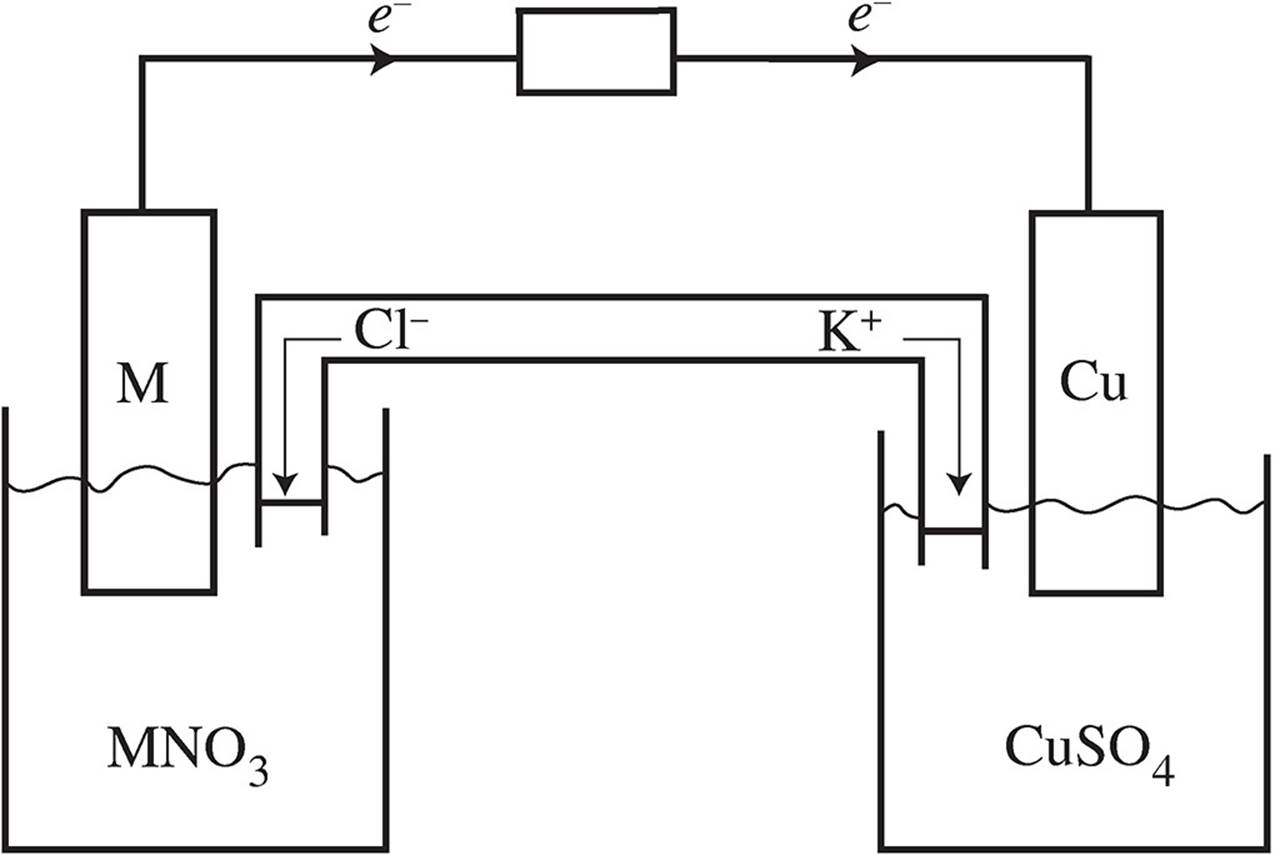
Electrons should be flowing from metal M to the copper bar. In the salt bridge, the K+ ions will flow towards the copper solution to replace the Cu2+ being reduced into Cu(s). The Cl− will flow towards the solution for metal M in order to balance out the charge of the extra M+ions being created via the oxidation of M(s).
(e) If the concentration of the Cu2+ increases, it will cause a shift to the right in accordance with Le Châtelier’s principle. This will increase the cell’s overall voltage.
6. (a) The two potential reduction reactions are the bottom two, as their reactants (H2O and Ni2+) are actually present at the start of the reaction. Of the two, the nickel reduction is more positive, and thus will take place. The top two reactions must be flipped to have the reactants (H2O and F−) on the reactants side, which also flips the sign. the sign the water oxidation will have a more positive value and thus will occur.
Reduction: Ni2+ + 2e− → Ni(s)
Oxidation: H2O(l) → O2(g) + 4H+ + 4e−
After multiplying the reduction half reaction by 2 to balance the electrons and combining both half reactions, the net cell reaction is:
2Ni2+ + H2O(l) → 2Ni(s) + O2(g) + 4H+
(b) At the cathode, solid nickel would begin to plate out of solution. At the anode, oxygen gas would form and bubble up to the surface.
(c) The solution will become more acidic due to the creation of hydrogen ions at the anode
(d) 1.20 g Ni × 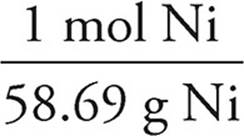 ×
× 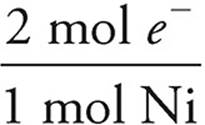 ×
× 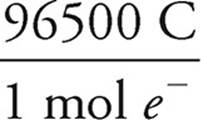 ×
× 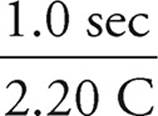 = 1790 seconds
= 1790 seconds