5 Steps to a 5: AP Chemistry 2020 - Moore J.T., Langley R.H. 2020
STEP 4 Review the Knowledge You Need to Score High
CHAPTER 8 Gases
IN THIS CHAPTER
Summary: Of the three states of matter—gases, liquids, and solids—gases are probably the best understood and have the best descriptive model. While studying gases in this chapter you will consider four main physical properties—volume, pressure, temperature, and amount—and their interrelationships. These relationships, commonly called gas laws, show up quite often on the AP exam, so you will spend quite a bit of time working problems in this chapter. But before we start looking at the gas laws, let’s look at the Kinetic Molecular Theory of Gases, the extremely useful model that scientists use to represent the gaseous state.

Keywords and Equations
Gas constant, R = 0.0821 L atm mol−1 K−1
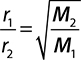
urms = root mean square speed
r = rate of effusion
STP = 0.000°C and 1.000 atm
PV = nRT
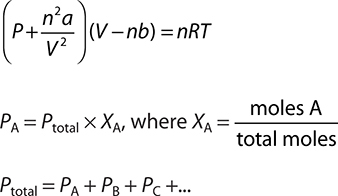
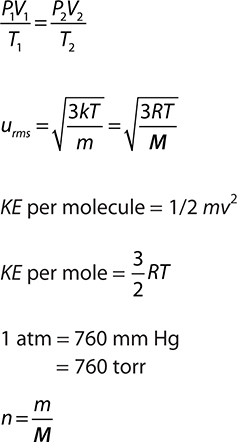
Kinetic Molecular Theory

The Kinetic Molecular Theory attempts to represent the properties of gases by modeling the gas particles themselves at the microscopic level. There are five main postulates of the Kinetic Molecular Theory:
1. Gases are composed of very small particles, either molecules or atoms.
2. The gas particles are tiny in comparison to the distances between them, so we assume that the volume of the gas particles themselves is negligible.
3. These gas particles are in constant motion, moving in straight lines in a random fashion and colliding with each other and the inside walls of the container. The collisions with the inside container walls comprise the pressure of the gas.
4. The gas particles are assumed to neither attract nor repel each other. They may collide with each other, but if they do, the collisions are assumed to be elastic. No kinetic energy is lost, only transferred from one gas molecule to another.
5. The average kinetic energy of the gas is proportional to the Kelvin temperature.
A gas that obeys these five postulates is an ideal gas. However, just as there are no ideal students, there are no ideal gases: only gases that approach ideal behavior. We know that real gas particles do occupy a certain finite volume, and we know that there are interactions between real gas particles. These factors cause real gases to deviate a little from the ideal behavior of the Kinetic Molecular Theory. But, a non-polar gas at a low pressure and high temperature would come pretty close to ideal behavior. Later in this chapter, we’ll show how to modify our equations to account for non-ideal behavior.
Before we leave the Kinetic Molecular Theory (KMT) and start examining the gas law relationships, let’s quantify a couple of the postulates of the KMT. Postulate 3 qualitatively describes the motion of the gas particles. The average velocity of the gas particles is called the root mean square speed and is given the symbol urms. This is a special type of average speed. It is the speed of a gas particle having the average kinetic energy of the gas particles. Mathematically it can be represented as:
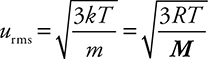
where R is the molar gas constant (we’ll talk more about it in the section dealing with the ideal gas equation), T is the Kelvin temperature, and M is the molar mass of the gas. These root mean square speeds are very high. Hydrogen gas, H2, at 20°C has a value of approximately 2,000 m/s.
Postulate 5 relates the average kinetic energy of the gas particles to the Kelvin temperature. Mathematically we can represent the average kinetic energy per molecule as:
KE per molecule = 1/2 mv2
where m is the mass of the molecule and v is its velocity.
The average kinetic energy per mol of gas is represented by:
KE per mol = 3/2 RT
where R again is the ideal gas constant and T is the Kelvin temperature. This shows the direct relationship between the average kinetic energy of the gas particles and the Kelvin temperature.
Gas Law Relationships
The gas laws relate the physical properties of volume, pressure, temperature, and moles (amount) to each other. First we will examine the individual gas law relationships. You will need to know these relationships for the AP exam, but the use of the individual equation is not required. Then we will combine the relationships into a single equation that you will need to be able to apply. But first, we need to describe a few things concerning pressure.
Pressure
When we use the word pressure, we may be referring to the pressure of a gas inside a container or to atmospheric pressure, the pressure due to the weight of the atmosphere above us. These two different types of pressure are measured in slightly different ways. Atmospheric pressure is measured using a barometer (Figure 8.1).
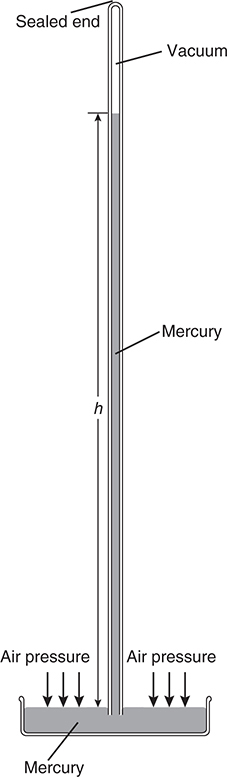
Figure 8.1 The mercury barometer.
An evacuated hollow tube sealed at one end is filled with mercury, and then the open end is immersed in a pool of mercury. Gravity will tend to pull the liquid level inside the tube down, while the weight of the atmospheric gases on the surface of the mercury pool will tend to force the liquid up into the tube. These two opposing forces will quickly balance each other, and the column of mercury inside the tube will stabilize. The height of the column of mercury above the surface of the mercury pool is called the atmospheric pressure. At sea level, the column averages 760 mm high. This pressure is also called 1 atmosphere (atm). Commonly, the unit torr is used for pressure, where 1 torr = 1 mm Hg, so that atmospheric pressure at sea level equals 760 torr. The SI unit of pressure is the pascal (Pa), so that 1 atm = 760 mm Hg = 760 torr = 101,325 Pa (101.325 kPa). In the United States pounds per square inch (psi) is sometimes used, so that 1 atm = 14.69 psi.
To measure the gas pressure inside a container, a manometer (Figure 8.2) is used. As in the barometer, the pressure of the gas is balanced against a column of mercury.
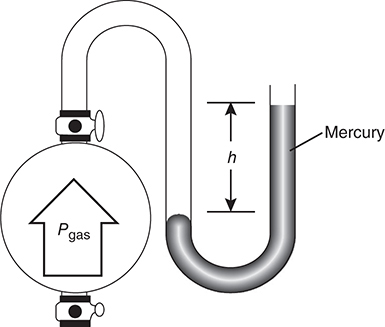
Figure 8.2 The manometer.
Volume—Pressure Relationship: Boyle’s Law

Boyle’s law describes the relationship between the volume and the pressure of a gas when the temperature and amount are constant. If you have a container like the one shown in Figure 8.3 and you decrease the volume of the container, the pressure of the gas increases because the number of collisions of gas particles with the container’s inside walls increases.
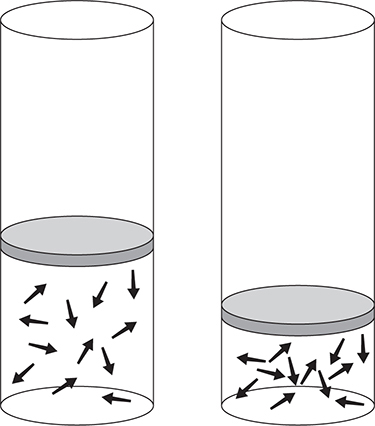
Figure 8.3 Volume—pressure relationship for gases. As the volume decreases, the number of collisions increases.
Mathematically this is an inverse relationship, so the product of the pressure and volume is a constant: PV = kb.
If you take a gas at an initial volume (V1) and pressure (P1) (amount and temperature constant) and change the volume (V2) and pressure (P2), you can relate the two sets of conditions to each other by the equation:
P1V1 = P2V2
In this mathematical statement of Boyle’s law, if you know any three quantities, you can calculate the fourth.
Volume—Temperature Relationship: Charles’s Law

Charles’s law describes the volume and temperature relationship of a gas when the pressure and amount are constant. If a sample of gas is heated, the volume must increase for the pressure to remain constant. This is shown in Figure 8.4.
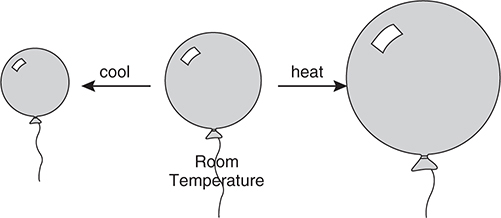
Figure 8.4 Volume—temperature relationship for gases.

Remember: In any gas law calculation, you must express the temperature in kelvin.
There is a direct relationship between the Kelvin temperature and the volume: as one increases, the other also increases. Mathematically, Charles’s law can be represented as:
V/T = kc
where kc is a constant and the temperature is expressed in kelvin.
Again, if there is a change from one set of volume—temperature conditions to another, Charles’s law can be expressed as:
V1/T1 = V2/T2
Pressure—Temperature Relationship: Gay-Lussac’s Law
Gay-Lussac’s law describes the relationship between the pressure of a gas and its Kelvin temperature if the volume and amount are held constant. Figure 8.5 represents the process of heating a given amount of gas at a constant volume.
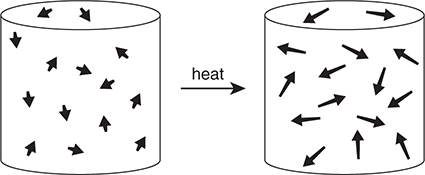
Figure 8.5 Pressure—temperature relationship for gases. As the temperature increases, the gas particles have greater kinetic energy (longer arrows) and collisions are more frequent and forceful.
As the gas is heated, the particles move with greater kinetic energy, striking the inside walls of the container more often and with greater force. This causes the pressure of the gas to increase. The relationship between the Kelvin temperature and the pressure is a direct one:
P/T = kg or P1/T1 = P2/T2
Combined Gas Law

In the discussion of Boyle’s, Charles’s, and Gay-Lussac’s laws, we held two of the four variables constant, changed the third, and looked at its effect on the fourth variable. If we keep the number of moles of gas constant—that is, no gas can get in or out—then we can combine these three gas laws into one, the combined gas law, which can be expressed as:
(P1V1)/T1 = (P2V2)/T2

Again, remember: In any gas law calculation, you must express the temperature in Kelvin.
In this equation, there are six unknowns; given any five, you should be able to solve for the sixth.
For example, suppose a 5.0-L bottle of gas with a pressure of 2.50 atm at 20°C is heated to 80°C. We can calculate the new pressure using the combined gas law. Before we start working mathematically, however, let’s do some reasoning. The volume of the bottle hasn’t changed, and neither has the number of moles of gas inside. Only the temperature and pressure have changed, so this is really a Gay-Lussac’s law problem. From Gay-Lussac’s law you know that if you increase the temperature, the pressure should increase if the amount and volume are constant. This means that when you calculate the new pressure, it should be greater than 2.50 atm; if it is less, you’ve made an error. Also, remember that the temperatures must be expressed in kelvin. 20°C = 293 K (K = °C + 273) and 80°C = 353 K.
We will be solving for P2, so we will take the combined gas law and rearrange for P2:
(T2P1V1)/(T1V2) = P2
Substituting in the values:
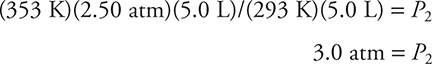
The new pressure is greater than the original pressure, making the answer a reasonable one. Note that all the units canceled except atm, which is the unit that you wanted.
Let’s look at a situation in which two conditions change. Suppose a balloon has a volume at sea level of 10.0 L at 760.0 torr and 20°C (293 K). The balloon is released and rises to an altitude where the pressure is 450.0 torr and the temperature is −10°C (263 K). You want to calculate the new volume of the balloon. You know that you have to express the temperature in K in the calculations. It is perfectly fine to leave the pressures in torr. It really doesn’t matter what pressure and volume units you use, as long as they are consistent in the problem. The pressure is decreasing, so that should cause the volume to increase (Boyle’s law). The temperature is decreasing, so that should cause the volume to decrease (Charles’s law). Here you have two competing factors, so it is difficult to predict the end result. You’ll simply have to do the calculations and see.
Using the combined gas equation, solve for the new volume (V2):
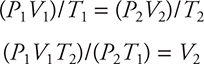
Now substitute the known quantities into the equation. (You could substitute the knowns into the combined gas equation first, and then solve for the volume. Do it which-ever way is easier for you.)
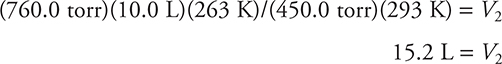
Note that the units canceled, leaving the desired volume unit of liters. Overall, the volume did increase, so in this case the pressure decrease had a greater effect than the temperature decrease. This seems reasonable, looking at the numbers. There is a relatively small change in the Kelvin temperature (293 K versus 263 K) compared to a much larger change in the pressure (760.0 torr versus 450.0 torr).
Volume—Amount Relationship: Avogadro’s Law

In all the gas law problems so far, the amount of gas has been constant. But, what if the amount changes? That is where Avogadro’s law comes into play.
If a container is kept at constant pressure and temperature, and you increase the number of gas particles in that container, the volume will have to increase in order to keep the pressure constant. This means that there is a direct relationship between the volume and the number of moles of gas (n). This is Avogadro’s law and mathematically it looks like this:
V/n = ka or V1/n1 = V2/n2
We could work this into the combined gas law, but more commonly the amount of gas is related to the other physical properties through another relationship that Avogadro developed:
1 mol of any gas occupies 22.4 L at STP
[standard temperature and pressure of 0°C (273 K) and 1 atm]
The combined gas law and Avogadro’s relationship can then be combined into the ideal gas equation, which incorporates the pressure, volume, temperature, and amount relationships of a gas.
Ideal Gas Equation
The ideal gas equation has the mathematical form of PV = nRT, where:

P = pressure of the gas in atm, torr, mm Hg, Pa, etc.
V = volume of the gas in L, mL, etc.
n = number of moles of gas
R = ideal gas constant: 0.0821 L·atm/K·mol
T = Kelvin temperature
This is the value for R if the volume is expressed in liters, the pressure in atmospheres, and the temperature in Kelvin (naturally). You could calculate another ideal gas constant based on different units of pressure and volume, but the simplest thing to do is to use the 0.0821 and convert the given volume to liters and the pressure to atm. And remember that you must express the temperature in kelvin.
Let’s see how we might use the ideal gas equation. Suppose you want to know what volume 20.0 g of hydrogen gas would occupy at 27°C and 0.950 atm. You have the pressure in atm, you can get the temperature in kelvin (27°C + 273 = 300.K), but you will need to convert the grams of hydrogen gas to moles of hydrogen gas before you can use the ideal gas equation. Also, remember that hydrogen gas is diatomic, H2.
First, you’ll convert the 20.0 g to moles:
(20.0 g/l) × (1 mol H2/2.016 g) = 9.921 mol H2
(We’re not worried about significant figures at this point, since this is an intermediate calculation.)
Now you can solve the ideal gas equation for the unknown quantity, the volume:
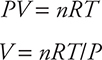
Finally, plug in the numerical values for the different known quantities:
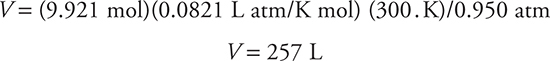
Is the answer reasonable? You have almost 10 mol of gas. It would occupy about 224 L at STP (10 mol × 22.4 L/mol) by Avogadro’s relationship. The pressure is slightly less than standard pressure of 1 atm, which would tend to increase the volume (Boyle’s law), and temperature is greater than standard temperature of 0°C, which would also increase the volume (Charles’s law). So you might expect a volume greater than 224 L, and that is exactly what you found.

Remember, the final thing you do when working any type of chemistry problem is answer this question: Is the answer reasonable?
Dalton’s Law of Partial Pressures

Dalton’s law says that in a mixture of gases (A + B + C …) the total pressure is simply the sum of the partial pressures (the pressures associated with each individual gas). Mathematically, Dalton’s law looks like this:
PTotal = PA + PB + PC + ⋯
Commonly Dalton’s law is used in calculations involving the collection of a gas over water, as in the displacement of water by oxygen gas. In this situation, there is a gas mixture: O2 and water vapor, H2O(g). The total pressure in this case is usually atmospheric pressure, and the partial pressure of the water vapor is determined by looking up the vapor pressure of water at the temperature of the water in a reference book. Simple subtraction generates the partial pressure of the oxygen.
If you know how many moles of each gas are in the mixture and the total pressure, you can calculate the partial pressure of each gas by multiplying the total pressure by the mole fraction of each gas:
PA = (PTotal)(XA)
where XA = mole fraction of gas A. The mole fraction of gas A would be equal to the moles of gas A divided by the total moles of gas in the mixture.
Graham’s Law of Diffusion and Effusion

Graham’s law defines the relationship of the speed of gas diffusion (mixing of gases due to their kinetic energy) or effusion (movement of a gas through a tiny opening) and the gases’ molecular mass. The lighter the gas, the faster is its rate of effusion. Normally this is set up as the comparison of the effusion rates of two gases, and the specific mathematical relationship is:
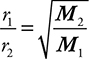
where r1 and r2 are the rates of effusion/diffusion of gases 1 and 2 respectively, and M2 and M1 are the molecular masses of gases 2 and 1 respectively. Note that this is an inverse relationship.
Figure 8.6 shows the Graham’s Law experimental set-up for NH3/HCl versus ND3/HCl. In the first experiment cotton balls saturated with NH3(aq) and HCl(aq) are inserted into a glass tube. The vapors travel through the tube and NH4Cl(s) formed where they meet.
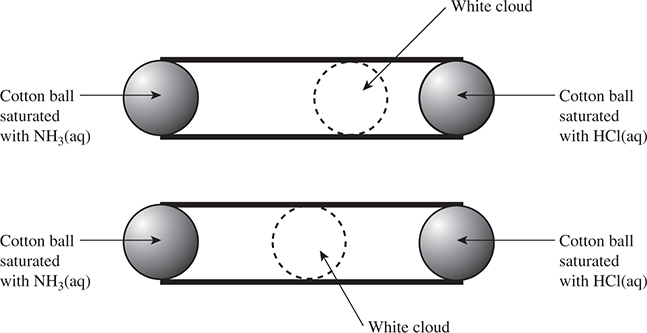
Figure 8.6 Graham’s Law of Diffusion set-up.
This forms the white cloud indicated in the figure. The experiment is repeated with ND3(aq) which is the form of ammonia where the hydrogens have been replaced with deuterium atoms. The molar mass of this compound is greater than ammonia and thus the vapors travel slower according to Graham’s Law. Therefore, the white cloud forms closer to the ND3 end than in the first experiment.
Suppose you wanted to calculate the ratio of effusion rates for hydrogen and nitrogen gases. Remember that both are diatomic, so the molecular mass of H2 is 2.016 g/mol and the molecular mass of N2 would be 28.02 g/mol. Substituting into the Graham’s law equation:
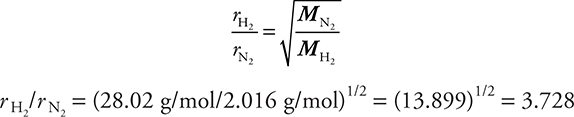
Hydrogen gas would effuse through a pinhole 3.728 times as fast as nitrogen gas. The answer is reasonable, since the lower the molecular mass, the faster the gas is moving. Sometimes we measure the effusion rates of a known gas and an unknown gas, and use Graham’s law to calculate the molecular mass of the unknown gas.
Gas Stoichiometry

The gas law relationships can be used in reaction stoichiometry problems. For example, suppose you have a mixture of KClO3 and NaCl, and you want to determine how many grams of KClO3 are present. You take the mixture and heat it. The KCIO3 decomposes according to the equation:
2 KClO3(s) → 2 KCl(s) + 3 O2(g)
The oxygen gas that is formed is collected by displacement of water. It occupies a volume of 542 mL at 27°C. The atmospheric pressure is 755.0 torr. The vapor pressure of water at 27°C is 26.7 torr.
First, you need to determine the pressure of just the oxygen gas. It was collected over water, so the total pressure of 755.0 torr is the sum of the partial pressures of the oxygen and the water vapor:
![]()
The partial pressure of water vapor at 27°C is 26.7 torr, so the partial pressure of the oxygen can be calculated by:
![]()
At this point, you have 542 mL of oxygen gas at 728.3 torr and 300.K (27°C + 273). From this data, you can use the ideal gas equation to calculate the number of moles of oxygen gas produced:
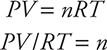
You will need to convert the pressure from torr to atm:
(728.3 torr) × (1 atm/760.0 torr) = 0.9583 atm
and express the volume in liters: 542 mL = 0.542 L
Now you can substitute the quantities into the ideal gas equation:
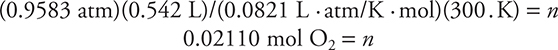
Now you can use the reaction stoichiometry to convert from moles O2 to moles KClO3 and then to grams KClO3:
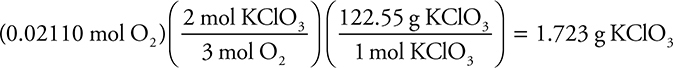
Non-Ideal Gases
We have been considering ideal gases, that is, gases that obey the postulates of the Kinetic Molecular Theory. But remember—a couple of those postulates were on shaky ground. The volume of the gas molecules was negligible, and there were no attractive forces between the gas particles. Many times approximations are fine and the ideal gas equation works well. But, it would be nice to have a more accurate model for doing extremely precise work or when a gas exhibits a relatively large attractive force. In 1873, Johannes van der Waals introduced a modification of the ideal gas equation that attempted to take into account the volume and attractive forces of real gases by introducing two constants—a and b—into the ideal gas equation. Van der Waals realized that the actual volume of the gas is less than the ideal gas because gas molecules have a finite volume. He also realized that the more moles of gas present, the greater the real volume. He compensated for the volume of the gas particles mathematically with:
corrected volume = V — nb
where n is the number of moles of gas and b is a different constant for each gas. The larger the gas particles, the more volume they occupy and the larger the b value.
The attraction of the gas particles for each other tends to lessen the pressure of the gas, because the attraction slightly reduces the force of gas particle collisions with the container walls. The amount of attraction depends on the concentration of gas particles and the magnitude of the particles’ intermolecular force. The greater the intermolecular forces of the gas, the higher the attraction is, and the less the real pressure. Van der Waals compensated for the attractive force with:
corrected pressure = P + an2/V2
where a is a constant for individual gases. The greater the attractive force between the molecules, the larger the value of a. The n2/V2 term corrects for the concentration. Substituting these corrections into the ideal gas equation gives the van der Waals equation:
(P + an2/V2)(V — nb) = nRT
The larger, more concentrated, and stronger the intermolecular forces of the gas, the more deviation from the ideal gas equation one can expect and the more useful the van der Waals equation becomes.
Experiments

Gas law experiments generally involve pressure, volume, and temperature measurements. In a few cases, other measurements such as mass and time are necessary. You should remember that ΔP, for example, is NOT a measurement; the initial and final pressure measurements are the actual measurements made in the laboratory. Another common error is the application of gas law type information and calculations for non-gaseous materials.
A common consideration is the presence of water vapor, H2O(g). Water generates a vapor pressure, which varies with the temperature. Dalton’s law is used in these cases to adjust the pressure of a gas sample for the presence of water vapor. The set-up for such an experiment is shown in Figure 8.7. As the substance in the test tube is heated, a gas is generated. That gas travels to the water-filled inverted test tube, it displaces the water, and the gas is collected. However, the gas inside the test tube is a mixture of gas from the decomposed substance and water vapor. The total pressure (normally atmospheric pressure) is the pressure of the gas or gases being collected and the water vapor. When the pressure of an individual gas is needed, the vapor pressure of water is subtracted from the total pressure. Finding the vapor pressure of water requires measuring the temperature and using a table showing vapor pressure of water versus temperature.
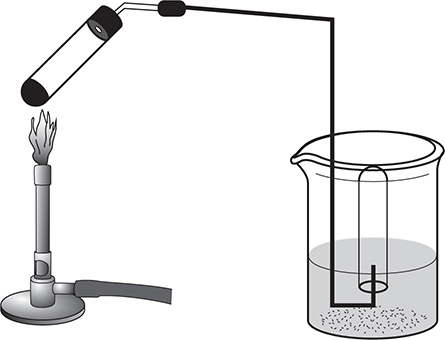
Figure 8.7 Dalton’s Law collection of a gas over water.
In experiments on Graham’s law, time is measured. The amount of time required for a sample to effuse is the measurement. The amount of material effusing divided by the time elapsed is the rate of effusion.
Most gas law experiments use either the combined gas law or the ideal gas equation. Moles of gas are a major factor in many of these experiments. The combined gas law can generate the moles of a gas by adjusting the volume to STP and using Avogadro’s relationship of 22.4 L/mol at STP. The ideal gas equation gives moles from the relationship n = PV/RT.
HINT: Make sure the conditions are STP before using 22.4 L/mol.
Two common gas law experiments are “Determination of Molar Mass by Vapor Density” and “Determination of the Molar Volume of a Gas.” While it is possible to use the combined gas law (through 22.4 L/mol at STP) for either of these, the ideal gas equation is easier to use. The values for P, V, T, and n must be determined.
The temperature may be determined easily using a thermometer. The temperature measurement is normally in °C. The °C must then be converted to a Kelvin temperature (K = °C + 273).
Pressure is measured using a barometer. If water vapor is present, a correction is needed in the pressure to compensate for its presence. The vapor pressure of water is found in a table of vapor pressure versus temperature. Subtract the value found in this table from the measured pressure (Dalton’s law). Values from tables are not considered to be measurements for an experiment. If you are going to use 0.0821 L atm/mol K for R, convert the pressure to atmospheres.
The value of V may be measured or calculated. A simple measurement of the volume of a container may be made, or a measurement of the volume of displaced water may be required. Calculating the volume requires knowing the number of moles of gas present. No matter how you get the volume, don’t forget to convert it to liters when using PV = nRT or STP.
The values of P, T, and V discussed above may be used, through the use of the ideal gas equation, to determine the number of moles present in a gaseous sample. Stoichiometry is the alternative method of determining the number of moles present. A quantity of a substance is converted to a gas. This conversion may be accomplished in a variety of ways. The most common stoichiometric methods are through volatilization or reaction. The volatilization method is the simplest. A weighed quantity (measure the mass) of a substance is converted to moles by using the molar mass (molecular weight). If a reaction is taking place, the quantity of one of the substances must be determined (normally with the mass and molar mass), and then, through the use of the mole-to-mole ratio, this value is converted to moles.
The values of P, T, and n may be used to determine the volume of a gas. If this volume is to be used with Avogadro’s law of 22.4 L/mol, the combined gas law must be employed to adjust the volume to STP. This equation will use the measured values for P and T along with the calculated value of V. These values are combined with STP conditions (0°C [273.15 K] and 1.00 atm) to determine the molar volume of a gas.
Combining the value of n with the measured mass of a sample will allow you to calculate the molar mass of the gas.
Do not forget: Values found in tables and conversions from one unit to another are not experimental measurements.
Common Mistakes to Avoid
1. When using any of the gas laws, be sure you are dealing with gases, not liquids or solids. We’ve lost track of how many times we’ve seen people apply gas laws in situations in which no gases were involved.
2. In any of the gas laws, be sure to express the temperature in kelvin. Failure to do so is a quite common mistake.
3. Be sure, especially in stoichiometry problems involving gases, that you are calculating the volume, pressure, etc. of the correct gas. You can avoid this mistake by clearly labeling your quantities (moles of O2 instead of just moles).
4. Make sure your answer is reasonable. Analyze the problem; don’t just write a number down from your calculator. Be sure to check your number of significant figures.
5. If you have a gas at a certain set of volume/temperature/pressure conditions and the conditions change, you will probably use the combined gas equation. If moles of gas are involved, the ideal gas equation will probably be useful.
6. Make sure your units cancel.
7. In using the combined gas equation, make sure you group all initial-condition quantities on one side of the equals sign and all final-condition quantities on the other side.
8. Be sure to use the correct molecular mass for those gases that exist as diatomic molecules—H2, N2, O2, F2, Cl2, and Br2 and I2 vapors.
9. If the value 22.4 L/mol is to be used, make absolutely sure that it is applied to a gas at STP.
![]() Review Questions
Review Questions
Use these questions to review the content of this chapter and practice for the AP Chemistry exam. First are 20 multiple-choice questions similar to what you will encounter in Section I of the AP Chemistry exam. Following those is a long free-response question like the ones in Section II of the exam. To make these questions an even more authentic practice for the actual exam, time yourself following the instructions provided.
Multiple-Choice Questions
Answer the following questions in 30 minutes. You may not use a calculator. You may use the periodic table and the equation sheet at the back of this book.
1. A sample of argon gas is sealed in a container. The volume of the container is doubled. If the pressure remains constant, what happens to the absolute temperature?
(A) The temperature does not change.
(B) The temperature is halved.
(C) The temperature is doubled.
(D) The temperature is squared.
2. A sealed, rigid container is filled with three ideal gases: A, B, and C. The partial pressure of each gas is known. The temperature and volume of the system are known. What additional information is necessary to determine the masses of the gases in the container?
(A) the average distance traveled between molecular collisions
(B) the intermolecular forces
(C) the molar masses of the gases
(D) the total pressure
3. Two balloons are at the same temperature and pressure. One contains 14 g of nitrogen, and the other contains 20.0 g of argon. Which of the following is true?
(A) The density of the nitrogen sample is greater than the density of the argon sample.
(B) The average speed of the nitrogen molecules is greater than the average speed of the argon molecules.
(C) The average kinetic energy of the nitrogen molecules is greater than the average kinetic energy of the argon molecules.
(D) The volume of the nitrogen container is less than the volume of the argon container.
4. An experiment to determine the molar mass of a gas begins by heating a solid to produce a gaseous product. The gas passes through a tube and displaces water in an inverted, water-filled bottle. Which of the following may be determined after the experiment is completed?
(A) vapor pressure of water
(B) temperature of the displaced water
(C) barometric pressure in the room
(D) mass of the solid used
5. The true volume of a real gas is larger than that calculated from the ideal gas equation. This occurs because the ideal gas equation does not consider which of the following?
(A) the attraction between the molecules
(B) the shape of the molecules
(C) the volume of the molecules
(D) the mass of the molecules
6. A 1.00 mole sample of ammonia gas, NH3, is placed in a flask. Ammonia will be most nearly ideal under which of the following sets of conditions?
(A) 250 K and 0.1 atm
(B) 350 K and 0.1 atm
(C) 350 K and 1 atm
(D) Ammonia will behave the same under all conditions.
7. A reaction produces a gaseous mixture of carbon dioxide, carbon monoxide, and water vapor. After one experiment, the mixture was analyzed and found to contain 0.60 mole of carbon dioxide, 0.30 mole of carbon monoxide, and 0.10 mole of water vapor. If the total pressure of the mixture was 0.80 atm, what was the partial pressure of the carbon monoxide?
(A) 0.080 atm
(B) 0.34 atm
(C) 0.13 atm
(D) 0.24 atm
8. A sample of methane gas was collected over water at 35°C. The sample had a total pressure of 756 mm Hg. Determine the partial pressure of the methane gas in the sample. The vapor pressure of water at 35°C is 41 mm Hg.
(A) 760 mm Hg
(B) 41 mm Hg
(C) 715 mm Hg
(D) 797 mm Hg
9. A student has three identical 2.0 L flasks (A, B, and C) all at 298 K. Each flask has an 8.0 g sample of gas sealed inside. Flask A contains methane, CH4; flask B contains hydrogen, H2; and flask C contains helium, He. Rank the three flasks in order of decreasing pressure.
(A) A > B > C
(B) B > C > A
(C) C > A > B
(D) All three flasks are at the same pressure.
10. An ideal gas sample weighing 1.28 g at 127°C and 1.00 atm has a volume of 0.250 L. Determine the molar mass of the gas.
(A) 322 g/mole
(B) 168 g/mole
(C) 0.00621 g/mole
(D) 80.5 g/mole
11. Increasing the temperature of an ideal gas from 50°C to 75°C at constant volume will cause which of the following to increase for the gas?
(A) the average molecular mass of the gas
(B) the average distance between the molecules
(C) the average speed of the molecules
(D) the density of the gas
12. If a sample of CH4 effuses at a rate of 9.0 moles per hour at 35°C, which of the following gases will effuse at approximately twice the rate under the same conditions?
(A) CO
(B) He
(C) O2
(D) F2
13. A steel tank containing argon gas has additional argon gas pumped into it at constant temperature. Which of the following is true for the gas in the tank?
(A) There is no change in the number of gas atoms.
(B) There is an increase in the volume of the gas.
(C) There is a decrease in the pressure exerted by the gas.
(D) The gas atoms travel with the same average speed.
14. Choose the gas that probably shows the greatest deviation from ideal gas behavior under a given set of conditions.
(A) He
(B) O2
(C) SF4
(D) SiH4
15. Determine the formula for a gaseous silane (SinH2n+2) if it has a density of 5.47 g/L at 0°C and 1.00 atm.
(A) SiH4
(B) Si2H6
(C) Si3H8
(D) Si4H10
16. Which of the following best explains why a hot air balloon rises?
(A) The heating of the air causes the pressure inside the balloon to increase.
(B) The moving outside air pushes the balloon higher.
(C) The temperature difference between the inside and outside air causes convection currents.
(D) Hot air has a lower density than cold air.
17. Three identical steel containers at the same temperature are filled with gas samples. One container has 16 g of methane, CH4; another has 44.0 g of carbon dioxide, CO2; and the third has 146 g of sulfur hexafluoride, SF6. Pick the FALSE statement from the following list:
(A) The densities decrease in the following order: sulfur hexafluoride > carbon dioxide > methane.
(B) Each container has the same number of molecules.
(C) The pressure in each container is the same.
(D) The molecules in each container have the same average speed.
18. Which of the following places the gases in order of increasing deviation from ideal behavior?
(A) He < SO2 < CH4 < O2
(B) He < O2 < CH4 < SO2
(C) He < CH4 < O2 < SO2
(D) CH4 < O2 < He < SO2
19. Each of four 10.0 L containers is filled with a different noble gas (He, Ne, Ar, and Kr). Each container contains 0.5 mole of gas at 298 K. Assuming all four gases are behaving ideally, which of the following is the same for all four samples?
(A) average speed of the atoms
(B) density of the gas in the container
(C) All properties are the same for gases behaving ideally.
(D) average kinetic energy of the atoms
20. Each of four 5.0 L containers is filled with a different gas (He, CH4, O2, and CO2). Each container contains 0.75 mole of gas at 273 K. If one of the containers springs a small leak, which of the following will change in that container?
(A) moles, temperature, and pressure
(B) moles and pressure
(C) temperature and pressure
(D) moles and temperature
![]() Answers and Explanations
Answers and Explanations
1. C—This question relates to the combined gas law: 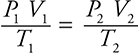 . Since the pressure remains constant, the pressures may be removed from the combined gas law to produce Charles’s law:
. Since the pressure remains constant, the pressures may be removed from the combined gas law to produce Charles’s law: 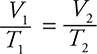 . This equation may be rearranged to
. This equation may be rearranged to 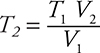 . The doubling of the volume means V2 = 2 V1. On substituting,
. The doubling of the volume means V2 = 2 V1. On substituting, 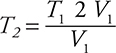 , giving T2 = 2 T1. The identity of the gas is irrelevant in this problem.
, giving T2 = 2 T1. The identity of the gas is irrelevant in this problem.
2. C—This problem depends on the ideal gas equation: PV = nRT. R, V, and T are known, and by using the partial pressure for a gas, the number of moles (n) of that gas may be determined. To convert from moles to mass, the molar mass of the gas is necessary.
3. B—Since T and P are known, and since the moles (n) can be determined from the masses given, this question could use the ideal gas equation. The number of moles of each gas is 0.50. Equal moles of gases, at the same T and P, have equal volumes, which eliminates answer choice D. Equal volume also means that the greater mass has the greater density, eliminating choice A. The average kinetic energy of a gas depends on the temperature. If the temperatures are the same, then the average kinetic energy is the same, eliminating C. Finally, at the same temperature, heavier gases travel slower than lighter gases. Nitrogen is lighter than argon, so it travels at a faster average speed, making B the correct answer. You may find this type of reasoning process beneficial on any question in which you do not immediately know the answer.
4. A—This experiment requires the ideal gas equation. The mass of the solid is needed (to convert to moles); this eliminates answer choice D. The volume, temperature, and pressure must also be measured during the experiment, eliminating choices B and C. The measured pressure is the total pressure. Eventually the total pressure must be converted to the partial pressure of the gas using Dalton’s law. The total pressure is the sum of the pressure of the gas plus the vapor pressure of water. The vapor pressure of water can be looked up in a table when the calculations are performed (only the temperature is needed to find the vapor pressure in a table). Answer A is correct because it is possible to delay looking up the vapor pressure of water.
5. C—Real gases are different from ideal gases because of two basic factors (see the van der Waals equation): molecules have a volume, and molecules attract each other. The molecules’ volume is subtracted from the observed volume for a real gas (giving a smaller volume), and the pressure has a term added to compensate for the attraction of the molecules (correcting for a smaller pressure). Since these are the only two directly related factors, answers B and D are eliminated. The question is asking about volume; thus, the answer is C.
6. B—A real gas approaches ideal behavior at higher temperatures and lower pressures.
7. D—The partial pressure of any gas is equal to its mole fraction times the total pressure. The mole fraction of carbon monoxide is 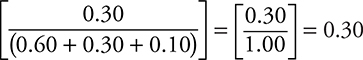 , and the partial pressure of CO is 0.30 × 0.80 atm = 0.24 atm.
, and the partial pressure of CO is 0.30 × 0.80 atm = 0.24 atm.
8. C—Using Dalton’s law (PTotal = PA + PB +…), where PA is unknown and PB is the vapor pressure of water, the partial pressure may be found by 756 mm Hg — 41 mm Hg = 715 mm Hg.
9. B—The molar masses of the gases are 2.0 g/mole for H2, 4.0 g/mole for He, and 16 g/mole for CH4. Therefore, an 8.0-g sample means 4.0 moles of H2, 2.0 moles of He, and 0.50 mole of CH4. The greater the number of moles present, with volume and temperature being the same, the greater the pressure in the flask.
10. B—The molar mass may be obtained by dividing the grams by the number of moles (calculated from the ideal gas equation). Estimation works in this case as 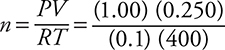 . Do not forget to convert the temperature to kelvin.
. Do not forget to convert the temperature to kelvin.
11. C—Choice B requires an increase in volume, not allowed by the problem. Choice C requires an increase in temperature. Choice A requires a change in the composition of the gas. Choice D requires a decrease in the volume.
12. B—Lighter gases effuse faster. The only gas among the choices that is lighter than methane is helium. To calculate the molar mass, you would begin with the molar mass of methane and divide by the rate difference squared.
13. D—A steel tank will have a constant volume, and the problem states that the temperature is constant. Adding gas to the tank will increase the number of moles of the gas and the pressure (forcing the argon atoms closer together). A constant temperature means there will be a constant average speed.
14. C—Deviations from ideal behavior depend on the size of the molecules and the intermolecular forces between the molecules. The greatest deviation would be for a large polar molecule. Sulfur tetrafluoride is the largest molecule, and it is the only polar molecule listed.
15. D—The molar mass of gas must be determined. The simplest method to find the molar mass is 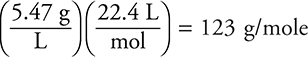 (simple factor label). The molar mass may also be determined by dividing the mass of the gas by the moles (using 22.4 L/mole for a gas at STP and using 1 L). If you did not recognize the conditions as STP, you could find the moles from the ideal gas equation. The correct answer is the gas with the molar mass closest to 123 g/mole.
(simple factor label). The molar mass may also be determined by dividing the mass of the gas by the moles (using 22.4 L/mole for a gas at STP and using 1 L). If you did not recognize the conditions as STP, you could find the moles from the ideal gas equation. The correct answer is the gas with the molar mass closest to 123 g/mole.
16. D—The hot air balloon rises because it has a lower density than the surrounding air. Less dense objects will float on more dense objects. In other words, “lighter” objects will float on “heavier” objects.
17. D—The densities come from the mass of gas divided by identical volumes; therefore, the container with the greatest mass of gas will have the greatest density. Each container holds one mole of gas, which means that each container has the same number of molecules of gas. The pressure in each container will be the same because the number of moles, the temperature, and the volume are the same. Ideal gases at the same temperature will have the same average kinetic energy. However, the heavier molecules do not need to travel as fast as the lighter molecules to attain equal kinetic energy. For this reason, the speeds are not identical.
18. C—The smaller the gas particle and the less polar (more nonpolar) the gas is, the smaller the deviation from ideal gas behavior. Sulfur dioxide, SO2, is the only polar gas; therefore, it will most likely show the greatest deviation. The remaining species (nonpolar) should be in order of increasing molar mass.
19. D—This behavior is due to kinetic molecular theory. The average kinetic energy depends only on the absolute temperature. The average speed changes with the atomic mass, with the heavier gas moving more slowly. Since the volumes are all the same, the higher the atomic mass, the higher the density.
20. A—All three variables will change, which makes this the best answer. The escaping gas would lead to a decrease in the number of moles. If there are fewer moles present, the pressure would decrease. The slower-moving gas molecules would tend to remain in the container, while the faster-moving molecules would escape. This would result in a lower average velocity of those species remaining in the container. If there is a lower average velocity, then there will be a lower temperature.
![]() Free-Response Question
Free-Response Question
You have 20 minutes to answer the following question. You may use a calculator and the tables in the back of the book.
Question
A sample containing 2/3 mol of potassium chlorate, KClO3, is heated until it decomposes to potassium chloride, KCl, and oxygen gas, O2. The oxygen is collected in an inverted bottle through the displacement of water. Answer the following questions using this information.
(a) Write a balanced chemical equation for the reaction.
(b) Calculate the number of moles of oxygen gas produced.
(c) The temperature and pressure of the sample are adjusted to STP. The volume of the sample is slightly greater than 22.4 L. Explain.
(d) An excess of sulfur, S, is burned in one mole of oxygen, in the presence of a catalyst, to form gaseous sulfur trioxide, SO3. Write a balanced chemical equation, and calculate the number of moles of gas formed.
(e) After the sulfur had completely reacted, a sample of the residual water was removed from the bottle and found to be acidic. Explain with a balanced chemical equation.
![]() Answer and Explanation
Answer and Explanation
(a) 2 KClO3(s) → 2 KCl(s) + 3 O2(g)
You get 1 point if you have the correct set-up of reactant and products. You get 1 more point if the equation is balanced correctly.
(b) (2/3 mol KClO3) (3 mol O2/2 mol KClO3) = 1 mol O2
You get 1 point for the correct answer and 1 point for the work. You can get these points if you correctly use information from an incorrect equation in part a.
(c) At STP, the volume of 1 mol of O2 should be 22.4 L. The volume is greater because oxygen was not the only gas in the sample. Water vapor was also present. The presence of the additional gas leads to a larger volume.
You get 1 point for discussing STP and 22.4 L, and 1 point for discussing the presence of water vapor.
(d) The equation is
2 S(s) + 3 O2(g) → 2 SO3(g)
According to this equation,
(1 mol O2) (2 mol SO3/3 mol O2) = 2/3 mol SO3
You get 1 point for the equation and 1 point for the math. You can get 1 total point if you used an incorrect number of moles of O2 from an incorrectly balanced equation.
(e) A nonmetal oxide, such as sulfur trioxide, will dissolve in water to produce an acid. This will get you 1 point. The following balanced chemical equation is worth 1 additional point:
SO3(g) + H2O(l) → H2SO4(aq)
Total your points. There are 11 possible points.
![]() Rapid Review
Rapid Review
• Kinetic Molecular Theory—Gases are small particles of negligible volume moving in a random straight-line motion, colliding with the container walls (that is the gas pressure) and with each other. During these collisions, no energy is lost, but energy may be transferred from one particle to another; the Kelvin temperature is proportional to the average kinetic energy. There is assumed to be no attraction between the particles.
• Pressure—Know how a barometer operates and the different units used in atmospheric pressure.
• Boyle’s law—The volume and pressure of a gas are inversely proportional if the temperature and amount are constant.
• Charles’s law—The volume and temperature of a gas are directly proportional if the amount and pressure are constant.
• Gay-Lussac’s law—The pressure and temperature of a gas are directly proportional if the amount and volume are constant.
• Combined gas law—Know how to use the combined gas equation: P1V1/T1 = P2V2/T2.
• Avogadro’s law—The number of moles and volume of a gas are directly proportional if the pressure and temperature are constant. Remember that 1 mol of an ideal gas at STP (1 atm and 0°C) occupies a volume of 22.4 L. Remember that you should not use the 22.4 L unless the gas is at STP.
• Ideal gas equation—Know how to use the ideal gas equation: PV = nRT.
• Dalton’s law—The sum of the partial pressures of the individual gases in a gas mixture is equal to the total pressure: PTotal = PA + PB + Pc +…
• Graham’s law—The lower the molecular mass of a gas, the faster it will effuse/diffuse. Know how to use Graham’s law: 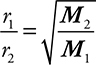 .
.
• Gas stoichiometry—Know how to apply the gas laws to reaction stoichiometry problems.
• Non-ideal gases—Know how the van der Waals equation accounts for the non-ideal behavior of real gases.
• Tips—Make sure the temperature is in Kelvin; gas laws are being applied to gases only; the units cancel; and the answer is reasonable.
• Gas laws are very useful for gases, but not for liquids and solids. Before applying a gas law, be sure you are dealing with a gas.