5 Steps to a 5: AP Chemistry 2020 - Moore J.T., Langley R.H. 2020
STEP 5 Build Your Test-Taking Confidence
AP Chemistry Practice Exam 1—Multiple Choice
ANSWER SHEET
The AP exam is a timed exam; keep this in mind as you prepare. When taking the various tests presented in this book, you should follow the AP exam rules as closely as possible. Anyone can improve his or her score by using notes, books, or an unlimited time. You will have none of these on the AP exam, so resist the temptation to use them on practice exams. Carefully time yourself, do not use other materials, and use a calculator only when expressly allowed to do so. After you have finished an exam, you may use other sources to go over questions you missed or skipped. We have seen many students get into trouble because the first time they attempted a test under “test conditions” was on the test itself.
AP Chemistry Practice Exam 1, Section I (Multiple Choice)
Time—1 hour and 30 minutes
NO CALCULATOR MAY BE USED WITH SECTION I
Answer the following questions in the time allowed. You may use the periodic table in the back of the book.
Use the following information to answer questions 1—7.
Sodium azide, NaN3, is a component of automobile airbags. It is useful because it quickly decomposes to generate a large volume of nitrogen gas. The balanced chemical equation for the reaction is:
2 NaN3(s) → 2 Na(s) + 3 N2(g)
There are additional components in the airbag to react with the elemental sodium formed.
Sodium azide is an unstable compound; therefore, it is often necessary to analyze samples as a check of its purity. A chemist is attempting to develop a new analytical, which employs the following reaction:
NaN3(aq) + Na2S(aq) + 3 H2O(l) → N2(g) + NH3(g) + S(s) + 3 NaOH(aq)
The chemist weighed a small flask both with and without a sample of sodium azide and recorded the masses. Next, she connected the flask to the system shown below.
The flask in the middle and the rubber tubing leading to the beaker were completely filled with dilute acid, and then the clamp was removed. Excess sodium sulfide solution was added to the flask containing the sample. The liquid level in the second flask dropped as the generated nitrogen gas displaced the water into the beaker. The system was left intact until gas generation ceased. After the system returned to room temperature, the beaker was raised until the water in the beaker was at the same level as in the second flask. When the liquid levels were the same, the clamp was replaced to prevent further transfer. The chemist completed the following data table in her lab book.
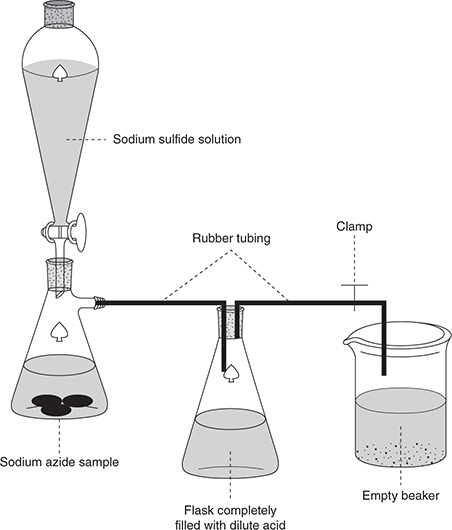

1. What type of reaction generated the nitrogen gas?
(A) Oxidation-reduction
(B) Combination
(C) Decomposition
(D) Combustion
2. Why must the liquid in the flask be dilute acid?
(A) Dilute acid is necessary to neutralize the sodium hydroxide formed.
(B) Dilute acid removes ammonia from the gas leaving the nitrogen behind.
(C) Dilute acid causes the reaction to go to completion.
(D) Dilute acid is easier to handle than many other liquids.
3. What is the partial pressure of the hydrogen gas in the flask?
(A) 775.2 torr
(B) 748.2 torr
(C) 760.0 torr
(D) 721.2 torr
4. After the system returned to room temperature an adjustment was made by raising the beaker until the liquid level in both the beaker and the flask were the same. Why was this step necessary?
(A) To remove excess water from the rubber tubing and into the beaker
(B) To equilibrate the pressure in the flask with the external pressure
(C) To make sure all of the hydrogen gas was out of the rubber tubing
(D) To make sure there was no contamination by the hydrochloric acid
5. Approximately, how many moles of nitrogen gas formed?
(A) 0.1 mole
(B) 0.02 mole
(C) 0.005 mole
(D) 0.01 mole
6. If the sample were pure sodium azide, approximately how many moles of nitrogen gas would form?
(A) 0.04 mole
(B) 0.002 mole
(C) 0.02 mole
(D) 0.2 mole
7. Would it be possible to use this experimental setup to study a reaction that produced gaseous sulfur dioxide, SO2? If not, why?
(A) No, because some of the sulfur dioxide gas would dissolve in the acid.
(B) No, because sulfur dioxide reacts with acids to produce solid sulfur.
(C) No, because sulfur dioxide is only a gas at very high temperatures.
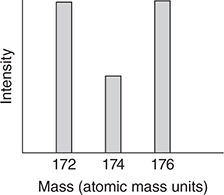
(D) Yes, this apparatus could be used.
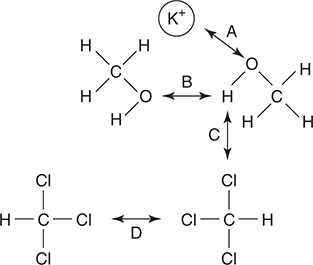
8. Which of the labeled arrows in the diagram above represents the strongest intermolecular force of the four indicated?
(A) Arrow A
(B) Arrow B
(C) Arrow C
(D) Arrow D
9. If about 88% of a sample of pure 131I decays in 24 days, what is the approximate half-life of 131I?
(A) 24 days
(B) 16 days
(C) 8 days
(D) 90 days
Use the following information for questions 10—12.

10. A buffered solution with a pH near 5 is needed for an experiment. Using the above information, which of the combinations would be the best choice to prepare the buffer?
(A) HIO3 + KIO3
(B) HCN + KCN
(C) HNO2 + KNO2
(D) HIO4 + NaIO4
11. A student wishes to measure the pH of a 0.10 M solution of the sodium salt of each of the acids in the table. These salts are NaIO3, NaIO4, NaNO2, and NaCN, respectively. Which of the four salt solutions will have the highest pH?
(A) NaIO3
(B) NaIO4
(C) NaNO2
(D) NaCN
12. Which of the acids in the table would be the easiest to titrate with a weak base like ammonia (Kb = 1.8 × 10—5)?
(A) HIO3
(B) HIO4
(C) HNO2
(D) HCN
Use the following information to answer questions 13−17.
pH versus volume of titrant added
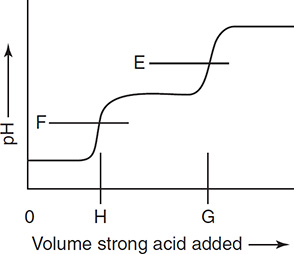
H2C6O6 Ka1 = 8.0 × 10−5 Ka2 = 1.6 × 10−12
Ascorbic acid (vitamin C), H2C6O6, is a diprotic acid used as a dietary supplement. As with all dietary supplements, it is important to analyze samples for purity. The vitamin C may be extracted from natural sources or synthesized. The extraction vitamin C may contain additional extracted ingredients to which some people are allergic. Synthetic vitamin C may contain other forms of the vitamin; however, modern synthetic methods do not generate this contaminate. The shown titration curve is an idealized graph for a diprotic acid. On this graph, E and F represent the pH at the endpoints with the possibility that E may shift slightly and one or the other may not be present. H is the volume of base required to titrate the first hydrogen ion and G is the quantity of base necessary to titrate both hydrogen ions. G is twice H.
13. What is the approximate pH at ½ H?
(A) 7
(B) 5
(C) 2
(D) Impossible to predict
14. If G were not twice H, what would this indicate?
(A) There is a contaminant that is either an acid or a base.
(B) The other form of vitamin C is present.
(C) The vitamin C was extracted from a plant.
(D) The vitamin C is synthetic.
15. Which of the following bases would be the best choice for the titration?
(A) Al(OH)3
(B) Na2CO3
(C) NH3
(D) KOH
16. In the titration of a sample of vitamin C, what is the approximate value of F?
(A) < 7
(B) > 7
(C) = 7
(D) Unknown
17. While the titration of a diprotic acid to produce a curve similar to the idealized one above is useful in many analyses, ascorbic acid is not a good candidate for this type of analysis. Why?
(A) Ka2 and Ka1 are too close together.
(B) Ascorbic acid may occur in more than one form.
(C) Ascorbic acid is not soluble in water.
(D) Ka2 for ascorbic acid is too small.
18. Which of the following CANNOT behave as both a Brønsted base and a Brønsted acid?
(A) H2PO42−
(B) CO32−
(C) HSO3−
(D) HCO3−
19. A student mixes 50.0 mL of 0.10 M potassium chromate, K2CrO4, solution with 50.0 mL of 0.10 M AgNO3. A red precipitate of silver chromate forms and the concentration of the silver ion becomes very small. Which of the following correctly places the concentrations of the remaining ions in order of decreasing concentration?
(A) [K+] > [CrO42−] > [NO3−]
(B) [CrO42−] > [NO3−] > [K+]
(C) [K+] > [NO3−] > [CrO42−]
(D) [NO3−] > [K+] > [CrO42−]
20. There are a number of experiments for the determination of the molecular mass of a gas. One method begins by heating a solid or liquid in a flask to produce a gaseous product. The gas passes through a tube and displaces water in an inverted, water-filled bottle. The mass of the starting material is measured, along with the volume of the displaced water and the temperature of the system. The pressure in the inverted water-filled bottle is equal to the external pressure. Once the barometric pressure has been recorded, what other information is needed to finish the experiment?
(A) The heat of formation of the gas
(B) The density of the water
(C) The mass of the displaced water
(D) The vapor pressure of the water
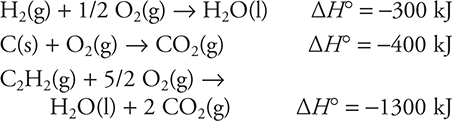
21. Using the information given above, calculate the enthalpy change for the following reaction:
2 C(s) + H2(g) → C2H2(g)
(A) 200 kJ
(B) −200 kJ
(C) 500 kJ
(D) −500 kJ
22. Cerium(III) sulfate, Ce2(SO4)2, is less soluble in hot water than it is in cold. Which of the following conclusions may be related to this?
(A) The heat of solution of cerium(III) sulfate is exothermic.
(B) The hydration energies of cerium ions and sulfate ions are very low.
(C) The heat of solution for cerium(III) sulfate is endothermic.
(D) The solution is not an ideal solution.
Use the information on the containers in the following diagram to answer questions 23—25.
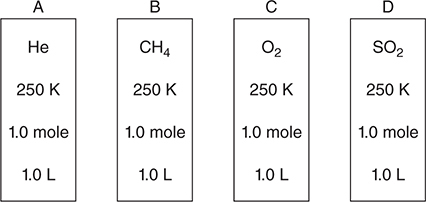
Approximate molar masses:
23. Under the conditions indicated, in which of the gas sample is the average velocity of the molecules half that of methane, CH4?
(A) He
(B) CH4
(C) SO2
(D) They are all at the same temperature; therefore, they have the same average velocity.
24. Which of the four gases will probably show the least deviation from ideal behavior?
(A) He
(B) CH4
(C) O2
(D) SO2
25. If one of the containers sprang a small leak, which of the following would change?
(A) Moles, temperature, and pressure
(B) Moles and pressure
(C) Temperature and pressure
(D) Moles and temperature
26. The specific rate constant, k, for a certain first-order reaction is 86 h−1. What mass of a 0.0500 g sample of starting material will remain after 58 s?
(A) 0.0500 g
(B) 0.0250 g
(C) 0.0125 g
(D) 0.00625 g
Use the information on the containers in the following diagram to answer questions 27−31 concerning the following equilibrium:
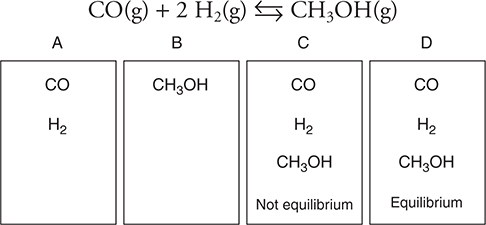
27. Container A initially contains 0.60 mole of H2 and 0.60 mole of CO and is allowed to come to equilibrium. At equilibrium, there are 0.40 mole of CO in the flask. What is the value of Kc, the equilibrium constant, for the reaction?
(A) 0.40
(B) 2.5
(C) 0.080
(D) 12
28. A 1.00-mole sample of CH3OH is placed in container B and the system is allowed to go to equilibrium. What can be said about the relative rates of reaction with respect to the various components?
(A) The rate of CO formation is numerically equal to the rate of CH3OH loss.
(B) The rate of H2 formation is numerically equal to the rate of CH3OH loss.
(C) The rate of H2(g) formation is half the rate of CO(g) formation.
(D) The rate of H2(g) formation is equal to the rate of CO(g) formation.
29. The mixture in container D is in equilibrium. Which of the following is true?
(A) The rate of the forward and reverse reactions is equal to zero.
(B) The rate of the forward reaction is equal to the rate of the reverse reaction.
(C) The pressure in the system is increasing.
(D) The pressure in the system is decreasing.
30. The mixture in container A goes to equilibrium. If the initial moles of H2(g) is twice the initial moles of CO(g), which of the following is true?
(A) Both reactants are limiting; therefore, the reaction will continue until there are zero moles of each remaining.
(B) The total pressure of the system decreases until the system reaches equilibrium.
(C) The total pressure of the system increases until the system equals equilibrium.
(D) No reaction occurs until a catalyst is added.
31. As the mixture in container B approaches equilibrium, the partial pressure of CH3OH gas decreases by 1.5 atm. What is the net change in the total pressure of the system?
(A) +1.5 atm
(B) +3.0 atm
(C) −1.5 atm
(D) −3.0 atm
Use the information on standard reduction potentials in the following table to answer questions 32−36.
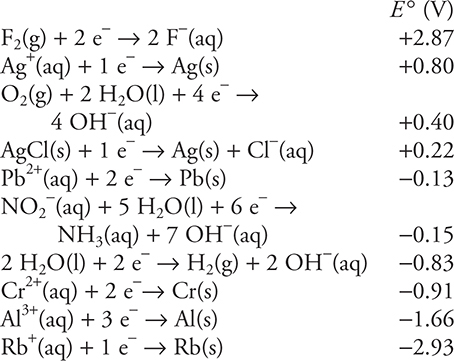
32. A student constructs an electrolysis cell with two inert electrodes in an aqueous solution that is 1.0 M in rubidium nitrite, RbNO2, and 1.0 M in rubidium hydroxide, RbOH. As the cell operates, an odorless gas evolves from one electrode and a gas with a distinctive odor evolves from the other electrode. Choose the correct statement from the following list.
(A) The odorless gas is oxygen.
(B) The odorless gas is the result of reduction.
(C) The gas with the distinctive odor is the result of oxidation.
(D) The odorless gas evolves at the negative electrode.
33. There is a galvanic cell involving a lead, Pb, electrode in a 1.0 M lead(II) nitrate, Pb(NO3)2, solution and a chromium, Cr, electrode in a 1.0 M chromium(II) sulfate, CrSO4, solution. What is the cell potential?
(A) +0.78 V
(B) −0.78 V
(C) +1.04 V
(D) 0.00 V
34. A student attempted to prepare an electrolysis cell to produce aluminum metal (Al) from an aqueous solution of aluminum chloride, AlCl3, using a 6.0 V battery. The cathode compartment of the electrolysis contained 1.0 M aluminum chloride. The student was unsuccessful. Why was the student unable to produce aluminum metal?
(A) The voltage from the battery was insufficient to force the reaction to occur.
(B) Reduction of chloride ion occurred in preference to reduction of calcium ion.
(C) Calcium chloride solutions do not conduct electricity.
(D) Reduction of water occurred in preference to reduction of calcium ion.
35. Which of the substances in the table would be capable of reducing the aluminum ions in solid aluminum fluoride, AlF3, to aluminum metal? Assume the cell potentials in the table also apply to the solid state.
(A) Cr(s)
(B) Rb(s)
(C) F2(g)
(D) None
36. A student constructs a galvanic cell that has a chromium, Cr, electrode in a compartment containing a 1.0 M chromium(II) nitrate, Cr(NO3)2, solution and a silver, Ag, electrode in a compartment containing 1.0 M silver nitrate, AgNO3, solution. A salt bridge containing a 1.0 M potassium chloride, KCl, solution connects the two compartments. When the student measures the cell potential, the value is far from the ideal predicted value. What is the cause of this discrepancy?
(A) The initial concentrations should have been lower than 1.0 M.
(B) The initial concentrations should have been higher than 1.0 M.
(C) The potassium chloride in the salt bridge interfered with the reaction.
(D) The student did not allow the cell to come to equilibrium.
Use the information on the acids in the following diagram to answer questions 37−38.
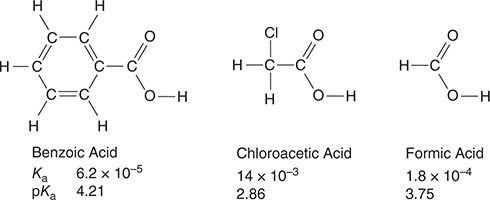
37. Sample solutions of each of the three acids were titrated with 0.10 M sodium hydroxide, NaOH. Each of the acid solutions had a concentration of 0.10 M. Which of the acid titrations had the highest pH at the endpoint?
(A) Formic acid
(B) Benzoic acid
(C) Chloroacetic acid
(D) They all had a pH of 7 at the endpoint.
38. A student prepares three buffer solutions. Each solution is 1.0 M in one of the acids in the table and 1.0 M in its corresponding sodium salt. Which of the solutions has the greatest buffer capacity with respect to added NaOH and why?
(A) The benzoic acid buffer because it is the strongest acid.
(B) The chloroacetic acid buffer because it is the strongest acid.
(C) The formic acid buffer because it donates both of its hydrogen atoms.
(D) All are the same.
39. Hypochlorous acid is an unstable compound, and one of the decomposition products is chlorine gas, Cl2. The decomposition of the acid lowers its concentration over time. What effect will the decomposition of one-fourth of the acid have on the agreement between the endpoint of the titration and the equivalence point during a titration with standard sodium hydroxide?
(A) The endpoint would still remain near the ideal equivalence point.
(B) The endpoint would be after the ideal equivalence point.
(C) The endpoint would be before the ideal equivalence point.
(D) It is impossible to determine.
40. Three 25.00 mL samples of approximately 0.10 M phenol, C6H5OH, Ka = 1.0 × 10−10 were removed from a container and placed in separate 250 mL beakers. The samples were titrated with standard potassium hydroxide, KOH, solution. Cresol red was the acid-base indicator used in the titration. The samples required 31.75, 32.38, and 41.75 mL to reach the endpoint. Which of the following might explain why one of the samples required significantly more base to reach the endpoint?
(A) The indicator was added too late.
(B) The wrong indicator was used.
(C) There was a base contaminating the unclean beaker.
(D) There was an acid contaminating the unclean beaker.
41. During the study of the reaction A → 2B, a chemist constructs several graphs. The graph of [A] versus time and the graph of ln [A] versus time both give a curved line; however, the graph of 1/[A] versus time and gives a straight line. This implies the rate law is
(A) Rate = k[A]
(B) Rate = k[A]2
(C) Rate = k[A]0
(D) Rate = k[A]−1
42. The photoelectron spectrum of carbon has three equally sized peaks. What peak is at the lowest energy?
(A) The 1s peak has the lowest energy.
(B) The 2s peak has the lowest energy.
(C) The 2p peak has the lowest energy.
(D) The 1p peak has the lowest energy.
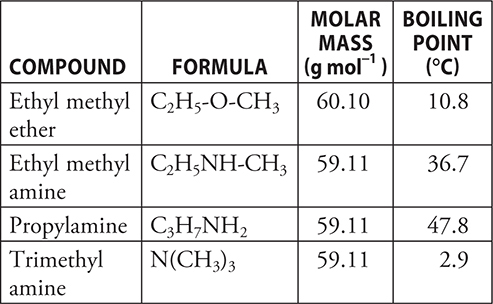
43. According to the data in the table above, which of the compounds has the strongest intermolecular forces?
(A) Propylamine
(B) Ethyl methyl ether
(C) Trimethyl amine
(D) Ethyl methyl amine
Use the following information to answer questions 44—45.
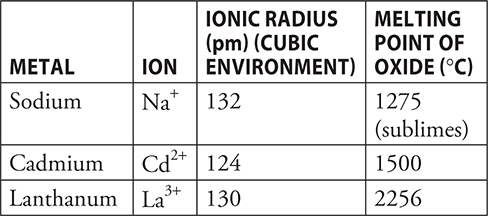
44. Each of the ions in the table form stable oxides (Na2O, CdO, and La2O3). Lanthanum oxide, La2O3, has a melting point significantly higher than that of the other oxides. Which of the following is the best explanation of why this is true?
(A) Lanthanum is a lanthanide element and the melting points of these elements are always high.
(B) There is more oxygen in the formula La2O3 than in the other formulas.
(C) Lanthanum had the highest charge; therefore, it has the highest lattice energy.
(D) Alkali metals like sodium and transition metals like cadmium tend to have low melting points.
45. The lithium ion, Li+, is smaller than the sodium ion. How does the melting point of lithium oxide, Li2O, compare to that of sodium oxide?
(A) It is higher because smaller ions have a higher lattice energy.
(B) It is the same because the charges are the same.
(C) It is lower because smaller ions have a smaller lattice energy.
(D) It is impossible to predict because there is insufficient information in the problem.
46. During the investigation of a chemical reaction by a chemistry student, she was able to determine that the reaction was nonspontaneous at 1 atm and 298 K. However, she learned that cooling the system with dry ice (−78°C) caused the reaction to become spontaneous. Which of the following combinations must apply to this reaction?
(A) ΔH < 0, ΔS < 0, and ΔG = 0
(B) ΔH > 0, ΔS < 0, and ΔG > 0
(C) ΔH < 0, ΔS < 0, and ΔG > 0
(D) ΔH > 0, ΔS > 0, and ΔG > 0
47. What is the ionization constant, Ka, for a weak monoprotic acid if a 0.060 molar solution has a pH of 2.0?
(A) 2.0 × 10−3
(B) 2.0 × 10−1
(C) 1.7 × 10−1
(D) 5.0 × 10−3
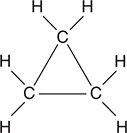
48. Cyclopropane, pictured above, is a relatively unstable compound. As seen in the diagram, the carbon atoms form the corners of an equilateral triangle and each carbon atom has two hydrogen atoms attached to complete an octet of electrons around the carbon atoms. Based upon this structure, why is cyclopropane a relatively unstable compound?
(A) Hydrocarbon compounds are relatively unstable in general.
(B) Compounds that have identical atoms bonded to each other are relatively unstable.
(C) The bonds do not match the angles.
(D) There is no resonance to stabilize the compound.
49. A chemist has a 500 mL beaker with a small amount of mercury(II) oxide, HgO, on the bottom. Mercury(II) is insoluble in water but will dissolve in strong acid. She adds 250 mL of water and begins adding 1.0 M hydrochloric acid. She continues adding acid until the solid just dissolves. She then tests the solution and finds that it is nonconducting. Which of the following best represents the result for the solution just as the last of the solid dissolves?
(A)
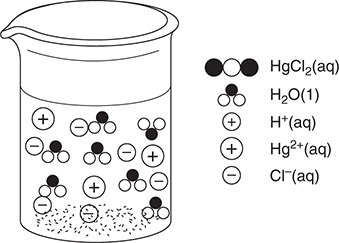
(B)
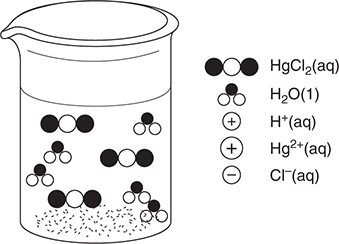
(C)
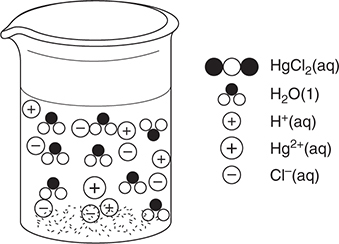
(D)
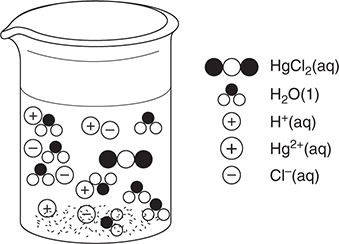
50. A dilute aqueous solution of potassium bromide, KBr, is heated to the boiling point. Which of the following best represents this system?
(A)
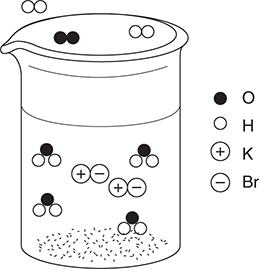
(B)
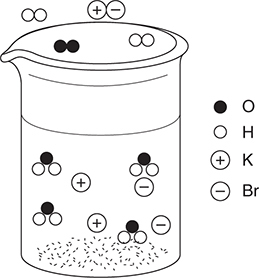
(C)
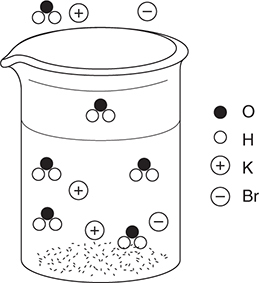
(D)
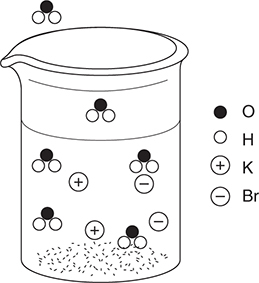
The average distribution of bromine isotopes on Earth is in the following table:

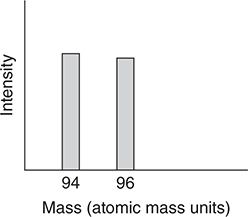
The highest mass peaks in the mass spectrum of methyl bromide, CH3Br, are at 94 and 96 atomic mass units (there are smaller peaks corresponding to the small quantities of the less common isotopes of carbon and hydrogen). The spectrum plot shown is of this region.
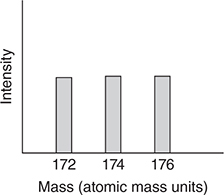
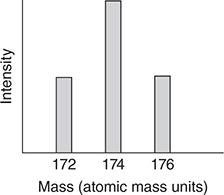
The mass spectrum for methylene bromide, CH2Br2, is more complicated in that there are three main peaks in the highest mass region (again ignoring minor contributions from other carbon and hydrogen isotopes). Methylene bromide has a mass of about 174 atomic mass units.
51. Which of the following is the best representation of the mass spectrum of CH2Br2 in the 174 region?
(A)
(B)
(C)
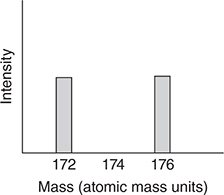
(D)
52. There are three steps in the formation of a solution. It is necessary to overcome the intermolecular forces present within the solute. It is also necessary to overcome the intermolecular forces present within the solvent. Both of these steps require energy related to the strength of the intermolecular forces. The final step in the formation of a solution involves the creation of new intermolecular forces between the solute and solvent. This energy release is related to the strength of the intermolecular forces created. Which of the following illustrates a situation most likely to require the least amount of energy to overcome the intermolecular forces?
(A) CaO(s) + H2O(l)
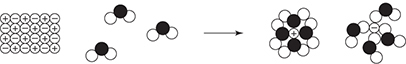
(B) CH3OH(l) + H2O(l)
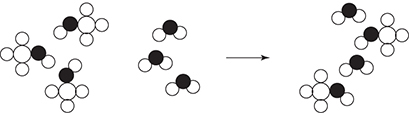
(C) CCl4(l) + Cl2(g)
(D) CCl4(l) + CH3OH(l)
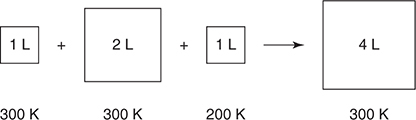
53. The contents in the three containers on the left in the diagram above are transferred to the container on the right. The volumes of the original containers are exactly the values indicated. The pressure in the first three containers is 1.0 atm. What is the pressure in the container on the right?
(A) 3.0 atm
(B) 4.0 atm
(C) 1.1 atm
(D) 0.50 atm
![]()
54. The diagram above shows the structure of molecules of CS2 and COS. The boiling point of COS is 223 K, and the boiling point of CS2 is 319 K. Which of the following is the best explanation of why the boiling point of CS2 is higher?
(A) The molar mass of CS2 is greater.
(B) COS has weaker covalent bonds than CS2.
(C) Only CS2 can form intermolecular dipole-dipole forces.
(D) COS has stronger intermolecular forces because it is polar and CS2 is not.
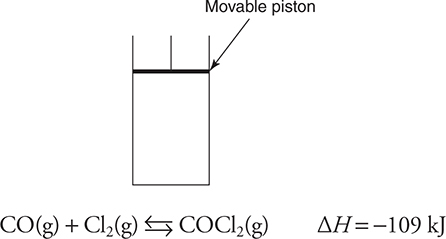
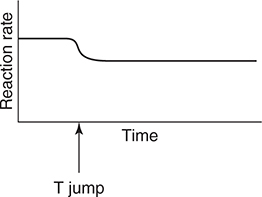
55. The above equilibrium is established in a closed system with a movable piston. After establishing equilibrium, the piston is rapidly moved up (pressure change). Which of the following graphs best illustrates the rate of the reverse reaction as the system returns to equilibrium?
(A)
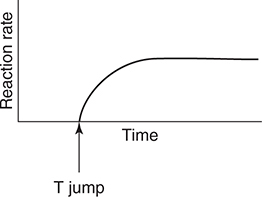
(B)
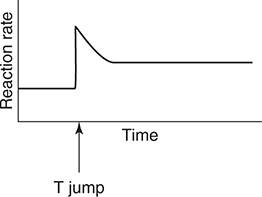
(C)
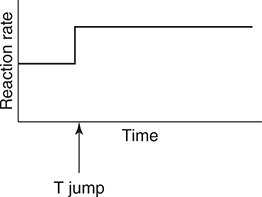
(D)
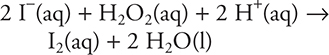
The proposed mechanism for the above reaction is as follows:
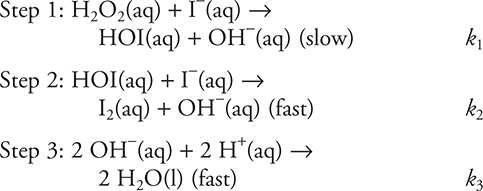
56. What is the rate law expression for this reaction?
(A) Rate = k[H2O2] [I−]
(B) Rate = k[HOI][I−]
(C) Rate = k[H2O2]
(D) 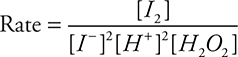
57. A 30.00 g sample of metal (X) was heated to 100.00°C. This sample was clamped in contact with a 40.00 g sample of a different metal (Y) originally at 25.00°C. The final temperature of the two metals was 37.30°C, and no heat was lost to the surroundings. What is one possible conclusion from this experiment?
(A) The heat lost by X was greater than the heat gained by the Y.
(B) The heat lost by X was equal to the heat gained by Y.
(C) The heat lost by X was less than the heat gained by Y.
(D) The final temperature was incorrectly determined, as it should be the average (62.50°C).
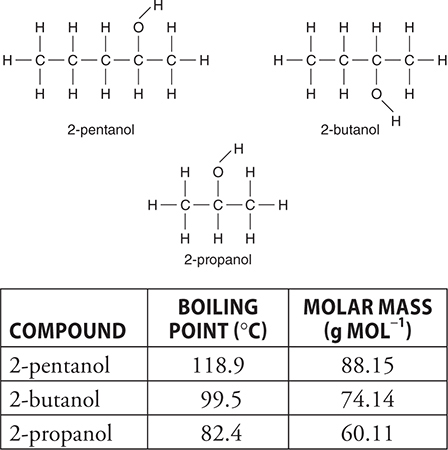
58. Which of the following best explains why the boiling point of 2-propanol is lower than the other two compounds in the diagram and table above?
(A) Larger molecules get tangled and cannot escape each other.
(B) It has weaker hydrogen bonds.
(C) It is the lightest of the three.
(D) It is a more symmetrical molecule.
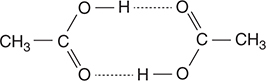
59. The Dumas method is a procedure for determining the molar mass of a gas. In this procedure the mass of a gas is divided by the moles of gas determined from the ideal gas equation (n = PV/RT). The molar masses of some compounds, such as acetic acid, illustrated above, show significant deviations from the “correct” values. Why does the presence of dimers as illustrated make it unlikely to obtain an accurate molar mass of acids, such as acetic acid?
(A) Acetic acid, like all acids, will lose a hydrogen ion, so the molar mass is that of the acetate ion, which is less than that of acetic acid.
(B) Acetic acid is a liquid at room temperature, and its boiling point is too high to get accurate results.
(C) Acids are too reactive to give accurate results.
(D) The presence of strong intermolecular forces (hydrogen bonding) makes the gas nonideal; therefore the ideal gas law is not applicable.
60. In which of the following groups are the species listed correctly in order of increasing ionization energy?
(A) Sr, Ca, Ba
(B) Se, Tc, Ba
(C) Mn, Fe, Ni
(D) Cl, Br, I
STOP. End of AP Chemistry Practice Exam 1, Section I (Multiple Choice).
![]() Answers and Explanations for Exam 1, Section 1 (Multiple Choice)
Answers and Explanations for Exam 1, Section 1 (Multiple Choice)
1. A—The reaction is an oxidation-reduction reaction since the sulfide ion undergoes oxidation to elemental sulfur and the azide ion undergoes reduction.
2. B—It is necessary to remove the ammonia from the gas; otherwise the part of the volume generated would be due to the ammonia. Since ammonia is a base, it will react with the acid.
3. D—The pressure inside the flask is the sum of the partial pressures. Therefore, the pressure of hydrogen gas is the total pressure (748.2 torr) minus the vapor pressure of water (27.0 torr). The leveling of the water in the beaker and flask adjusted the pressure in the flask to the external (barometric) pressure.
4. B—When the two liquid levels are the same then the pressures must be equal. In this case, both pressures are equal to the barometric pressure.
5. D—The ideal gas equation (PV = nRT) gives the moles of hydrogen gas formed.
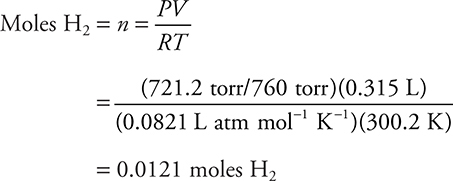
It is easier to calculate the answer by simple rounding as:
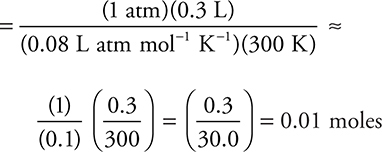
6. C—The moles of nitrogen gas formed equal the moles of sodium azide reacting (see the balanced chemical equation). If the sample were pure sodium azide, the mass of sodium azide would be (176.604 − 175.245) g = 1.359 g sample (= NaN3). The moles of sodium azide are the mass of sodium azide divided by its molar mass (65.0 g mol−1).
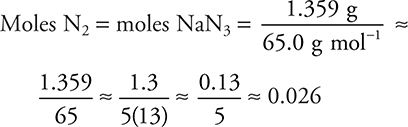
Due to rounding, the actual answer must be a little smaller (0.0209 moles).
7. A—Sulfur dioxide gas is soluble in water and, while less soluble in dilute acid, some would still dissolve to give a smaller volume.
8. A—This represents an ion-dipole force, which is stronger than a hydrogen bond (B), a dipole-dipole force (C), or a London dispersion force (D).
9. C—After one half-life, 50% would remain. Another half-life would reduce this by one-half to 25% and a third half-life would reduce the remaining material by 12.5%. Thus, three half-lives = (50 + 25 + 12.5)% = 87.5% decayed. The total amount decayed is 88%. For this reason, 24 days must be three half-lives of about 8 days each.
10. C—The best choice to prepare the buffer is the one where the pKa is closest to the desired pH. It is possible to estimate the pKa for an acid (without a calculator) by taking the negative of the exponent for the different Ka values. This gives 1 for HIO3, 2 for HIO4, 4 for HNO2, and 10 for HCN. The HNO2 has the value closest to the desired pH. It would not be a good choice since the pKa is so far from the pH; however, it is the best choice.
11. D—This is due to hydrolysis of the conjugate base of the acid (IO3—, IO4—, NO2—, and CN—). The smaller the Ka of the acid, the greater the Kb of the conjugate base. A larger Kb means a stronger base.
12. A—In order to get good results when titrating a weak base, it is important to use as strong an acid as possible to get a sharp endpoint. HIO3 is the strongest acid in the table.
13. B—The position ½ H is halfway to the first equivalence point. The pH at the halfway point is equal to pKa. The value of Ka1 is 8.0 × 10−5, which gives an approximate pKa of 5 (actual 4.1).
14. A—To reach H, it is necessary to convert all the ascorbic acid to the hydrogen ascorbate ion, which means the moles of hydrogen ascorbate ion formed must equal the moles of ascorbic acid originally present. The moles of hydrogen ascorbate ion formed would require the same number of moles of base as that required to convert the ascorbic acid to hydrogen ascorbate. Equal moles would mean equal volumes added.
15. D—In any acid-base titration, it is always easier to use a strong base (and a strong acid). Potassium hydroxide, KOH, is the only strong base among the choices.
16. B—The titration of a weak acid with a strong base always has an equivalence point above 7.
17. D—Since Ka2 is Ka2 = 1.6 × 10−12, the pH at the equivalence point would be greater than 12, which is too high for a simple titration.
18. B—All can behave as Brønsted bases (accept a hydrogen ion). Only B cannot behave as an acid (donate a hydrogen ion).
19. C—Initially, doubling the volume will result in halving the concentrations. Next, consider the reaction. The balanced equation is: K2CrO4(aq) + 2 AgNO3(aq) → Ag2CrO4(s) + 2 KNO3(aq). The silver ion is the limiting reagent, so very little remains in solution (due to its Ksp). The precipitation of silver chromate reduces the chromate concentration from 0.050 M. The nitrate does not change (soluble) remaining 0.050 M. Since two potassium ions (soluble) are formed per potassium chromate, after mixing the potassium ion concentration was 0.10 M and does not change (soluble).
20. D—To determine the molar mass of the gas it is necessary to know the mass of the gas (measured) and the moles of the gas. The ideal gas equation is necessary to determine the number of moles of gas present. To use the equation, it is necessary to know the temperature (measured), volume (measured = volume of displaced water), and pressure of the gas. The pressure of the gas is equal to the barometric pressure minus the vapor pressure of water. Water, whenever present, will contribute its vapor pressure.
21. A—
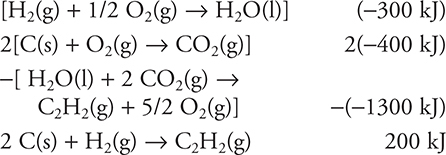
22. A—Since the compound is less soluble in hot water, the solution process must be exothermic. Exothermic processes shift toward the starting materials (solid cerium(III) sulfate) when heated.
23. C—The gases are all at the same temperature; therefore their average kinetic energies are the same. Since kinetic energy is equal to ½ mv2, ½ m1v12 = ½ m2v22, the subscripts refer to two different gases. Setting m1 = 16 g mol−1 and v2 = ½ v1 gives: ½ 16v12 = ½ m2(½ v1)2; this leads to: 16v12 = m2(1/4 v12). Rearranging and cancelling v1 yields m2 = 4(16) = 64 g mol−1.
24. A—The smaller the molecule and the less polar (more nonpolar) the gas is, the smaller the deviation from ideal gas behavior.
25. A—Escaping gas would decrease the number of moles. Less gas remaining in the container would mean less pressure. The faster moving gas molecules would escape faster, lower the average velocity of those remaining in the container. A lower average velocity means a lower temperature.
26. C—It is necessary to use the half-life relationship for first-order kinetics. This relationship is t1/2 = 0.693/k, and t1/2 = (0.693/86 h−1)(3600 s/h) = 29 s. To save time on the exam you can approximate this equation as t1/2 = (0.7/90)(3600). Dividing 3600 by 90 gives 40, and 40 times 0.7 is equal to 28. If the half-life is ≍ 28 s, then the time (58 s) is equivalent to about two half-lives, so one-fourth of the sample should remain.
27. D—The loss of 0.20 mol of CO means that 0.40 mol of H2 reacted (leaving 0.20 mol) and 0.20 mol of CH3OH formed. Dividing all the moles by the volume gives the molarity, and:
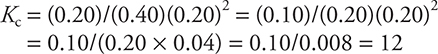
28. A—Based upon the stoichiometry of the reaction, H2(g) will form twice as fast as CH3OH(g) disappears. The numerical values are the same; however, the rates have opposite signs.
29. B—At equilibrium, there is no net change because the forward and reverse reactions are going at the same rate.
30. B—As the reaction approaches equilibrium, there is a net decrease in the number of moles of gas present. A decrease in the number of moles of gas will lead to a decrease in pressure
31. B—The loss of 1.5 atm of CH3OH(g), based on stoichiometry leads to the formation of 1.5 atm of CO(g) and 3.0 atm of H2(g); therefore, the net change is (−1.5 + 1.5 + 3.0) atm = 3.0 atm.
32. A—Oxygen (odorless) evolves at the anode (positive) and ammonia (distinctive odor) evolves at the cathode (negative). From the choices given, the only gas with a distinctive odor that could form is ammonia, NH3. For this reason, the nitrite ion half-reaction must be the cathode reaction. The anode half-reaction must be the reverse of one of the reactions in the table. The only one of the half-reactions given that generates an odorless gas is the one involving oxygen, O2.
33. A—The lead electrode is the cathode (−0.13 V), and the chromium electrode is the anode (reverse sign, +0.91 V). Cell voltage = (−0.13 + 0.91) V = +0.78 V. The standard voltage of a galvanic cell is always positive.
34. D—It is necessary to consider all possible half-reactions that might occur during electrolysis. Of the half-reactions listed, only the reduction of water and the reduction of aluminum ions are applicable to this experiment. For any electrolysis, the half-reaction requiring the least amount of energy will take place. So, while it is possible to reduce both water and the aluminum ion, the reduction potential for water is lower (requiring less energy), so water will reduce in preference to aluminum ion.
35. B—In order for substance A to reduce substance B, substance A must be a stronger reducing agent than substance B. Aluminum is a strong reducing agent as indicated by the large negative potential in the table (−1.66 V). The only substance in the table that has a more negative potential is rubidium, Rb (−2.93 V).
36. C—Silver appears twice in the list of half-reactions, once in the simple reduction of silver ions and once in the reduction of silver chloride, AgCl. The AgCl half-reaction clearly shows that this compound is a solid. This solid will form as the silver ion in the silver nitrate solution reacts with chloride ion from the potassium chloride in the salt bridge. The formation of solid silver chloride will alter the concentration of the silver ion, which leads to a change in the cell potential.
37. B—The weakest acid (smallest Ka) will have the highest pH at the endpoint.
38. D—The buffer capacity only depends on the number of moles present. All three solutions have the same number of moles.
39. C—Less acid would require less than the ideal amount of base to reach the endpoint. Therefore, the endpoint would occur too soon.
40. D—An acid contaminant was present and it would be necessary to add additional base to titrate this acid in addition to the phenol.
41. B—Since only the plot of 1/[A] versus time yielded a straight line, this implies that the reaction is second-order in A.
42. C—The electron configuration of carbon in 1s22s2sp2. The removal of the last electron (2p) requires the least amount of energy.
43. A—The compound with the highest boiling point has the strongest intermolecular forces. This is only valid if the molar masses are similar.
44. C—The melting points of ionic materials depend upon the lattice energy. The higher the lattice energy, the higher the melting point is. Lattice energies depend upon the sizes of the ions and the magnitude of the charge. All three metal ions are approximately the same size; therefore, the size factor is minimal. This leaves the magnitude of the charge, and lanthanum, with the largest charge, should have the highest lattice energy.
45. A—The lattice energy depends upon both the charge and the size of the ions involved. The greater the charge is, the greater the lattice energy will be (higher melting point). There is an inverse relationship between the lattice energy and the size of the ion. Therefore, the smaller the ion is, the greater the lattice energy will be (higher melting point).
46. C—If the reaction is nonspontaneous at 1 atm and 298 K, then ΔG > 0. For this reaction to become spontaneous at a lower temperature the reaction must be impeded by entropy (entropy was negative). The enthalpy must be negative or the reaction could never be spontaneous.
47. A—Enter the information into the Ka expression. A pH of 2.0 means that [H+] = 10−2.0 M or 1.0 × 10−2. Ka = [H+][A−]/[HA]; [H+] = [A−] = 1.0 × 10−2 [HA] = 0.060 − 1.0 × 10−2 = 0.05; therefore, Ka = (1.0 × 10−2)/0.050 = 0.002 = 2 × 10−3.
48. C—A carbon atom with four single bonds should be tetrahedral. Tetrahedral atoms have an ideal bond angle of 109.5°. However, the carbon atoms in cyclopropane are at the corners of an equilateral triangle, where the ideal angle is 60°. The discrepancy between the two ideal bond angles leads to the relative instability of cyclopropane.
49. B—The reaction is:
HgO(s) + 2 HCl(aq) → HgCl2(aq) + H2O(l)
When the last of the solid dissolves, we are at the stoichiometric point indicated by this reaction. No HgO remains and the chemist stopped adding HCl at this point, so there is no excess HCl present. The HgCl2 must be soluble because there is no solid remaining in the beaker. Since the resultant solution is nonconducting, there must be no electrolytes present; therefore, the products must be molecular species. The best answer should show only water molecules and HgCl2 molecules.
50. D—This answer shows the potassium ions and bromide ions as separate ions in solution, which is a property of strong electrolytes. The water molecules are present in both the liquid and gas states. Other diagrams incorrectly show water dissociating, potassium bromide ion pairs, and potassium bromide vaporizing.
51. B—There are two bromine isotopes with equal abundances, which is why there are two nearly equally intense peaks in the mass spectrum of CH3Br. One of the peaks corresponds to CH379Br and the other corresponds to CH381Br. The separation between the peaks is two mass units because the isotopic masses differ by two units. In the case of CH2Br2, the possible combinations and masses are CH279Br79Br (172), CH279Br81Br (174), CH281Br79Br (174), and CH281Br81Br (176). The four possible combinations are equally probable since the abundances of the two bromine isotopes are nearly equal. If they all had different masses, the mass spectrum would consist of four equally intense peaks. However, two of the combinations have the same mass, which results in the 174 peak being twice as intense as the other two peaks.
52. C—In diagram A, the forces to overcome are ionic bonding (lattice energy) and hydrogen bonding. In diagram B, the forces to overcome are hydrogen bonding for both the solvent and the solute. In diagram C, the forces to overcome are London dispersion forces in both cases; however, since the chlorine is a gas, the London dispersion forces have already been overcome. In diagram D, the forces to overcome are London dispersion forces and hydrogen bonding. Since London dispersion forces are normally weaker than the other intermolecular forces, answer C requires the least amount of energy.
53. C—There are several ways of solving this problem. One way is to determine the moles present in the original containers, which must be the same as in the final container. In each case, moles = n = PV/RT. Numbering the containers from left to right as 1, 2, 3, and 4 gives:
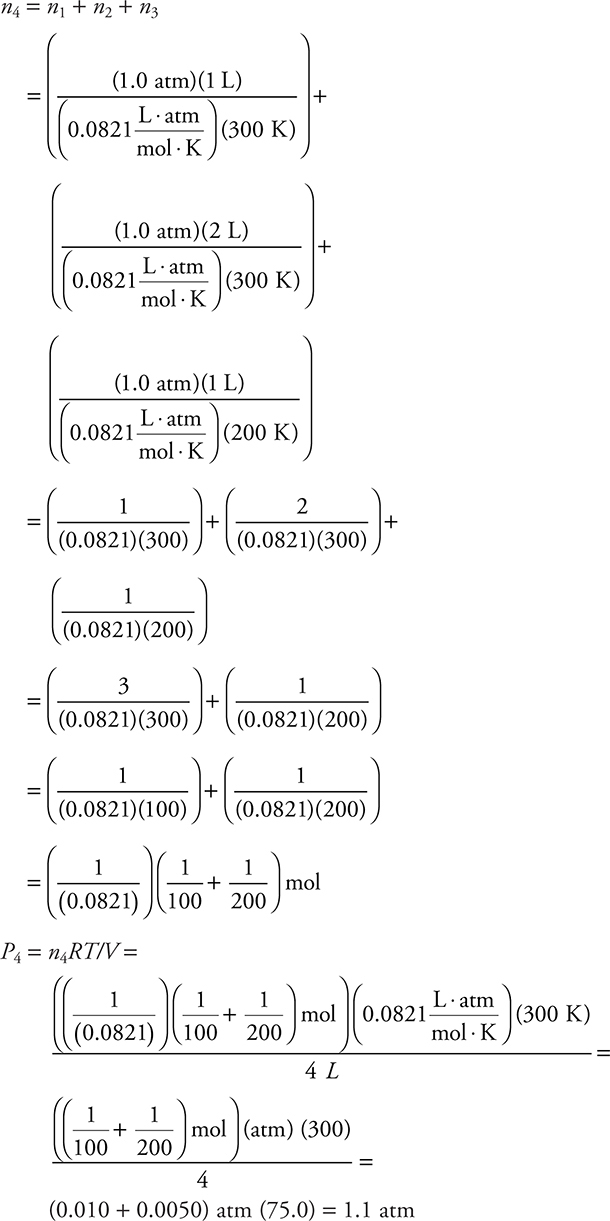
On the exam, it is not necessary to write out all these steps. Take shortcuts.
54. A—Stronger intermolecular forces lead to higher boiling points. Even though COS has dipole-dipole forces, which are usually stronger than the London dispersion forces present in CS2, the greater molar mass of CS2 leads to a London dispersion force contribution that is sufficient to compensate for the general trend of dipole-dipole forces being stronger than London dispersion forces. This is why comparisons should only be made between molecules of similar molecular masses.
55. B—At equilibrium the reverse reaction is going at a steady rate (equal to that of the forward reaction and not equal to 0). A sudden decrease in pressure will cause the rate of the reverse reaction to increase to generate more gas to increase the pressure. It will eventually slow and become constant again.
56. A—Only the slow step in a mechanism leads to the rate law. There is one H2O2 and one I− in the slow step; therefore, one of each of these will be in the rate law.
57. B—This must be true according to the Law of Conservation of Energy (First Law of Thermodynamics). Answers A and C violate the Law of Conservation of Energy. For the final temperature to be the average, the metals would need to have equal masses and equal specific heats. The masses given in the problem are clearly different, and it is extremely unlikely that two different metals would have the same specific heat.
58. C—All three compounds are capable of hydrogen bonding; therefore, this cannot be the cause of difference. In general, all other things being equal, it takes less energy to move a lighter molecule from the liquid state to the gaseous state.
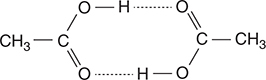
59. D—Strong hydrogen bonds hold two molecules of acetic acid together. Ideal gases have no intermolecular forces. Therefore, the ideal gas law used in experiment is invalid.
60. C—Increasing ionization energy applies to an element higher in a column on the periodic table, or in a position further to the left in a period on the periodic table. Note, this type of explanation is unacceptable on the Free Response portion of the AP Exam, where your explanation would require information on radii and effective nuclear charges.
![]() AP Chemistry Practice Exam 1, Section II (Free Response)
AP Chemistry Practice Exam 1, Section II (Free Response)
Time—1 hour and 45 minutes
Answer the following questions in the time allowed. You may use a calculator and the resources at the back of the book. Write the answers on separate sheets of paper.
Question 1
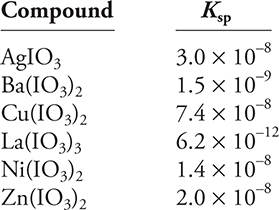
Use the Ksp data given above to answer the following questions.
A chemist is investing the chemistry of metal iodate compounds. Some of her data are in the Ksp table given above. In addition to this information, she knows that sodium iodate, NaIO3, is soluble in water and that iodic acid, HIO3, has Ka = 0.16 at 25°C.
(a) (i) Write a balanced chemical equation for the dissolution equilibrium of nickel(II) iodate, Ni(IO3)2, in water.
(ii) Write the Ksp relationship for the dissolution equilibrium of nickel(II) iodate.
(iii) What is the concentration of iodate ions in a saturated solution of nickel(II) iodate?
(b) What is the solubility of copper(II) iodate, Cu(IO3)2, in a 0.10 M copper(II) nitrate, Cu(NO3)2, solution?
(c) (i) How does the solubility of silver iodate, AgIO3, in 1.0 M nitric acid compare to its solubility in pure water?
(ii) Explain your answer to part c(i).
(d) Which of the following will produce a higher iodate ion concentration in solution, barium iodate, Ba(IO3)2, or lanthanum iodate, La(IO3)3? What is the iodate ion concentration for each of these two compounds?
(e) Write a balanced net ionic equation for the addition of a sodium iodate solution to a zinc nitrate, Zn(NO3)2 solution. Recall that sodium nitrate, NaNO3, is very soluble in water.
Question 2
Selenous acid, H2SeO3, reacts with hydrogen peroxide, H2O2, in acid solution according to the following equation:
H2SeO3(aq) + H2O2(aq) → HSeO4—(aq) + H+(aq) + H2O(l)
The following information was obtained in a series of reactions:
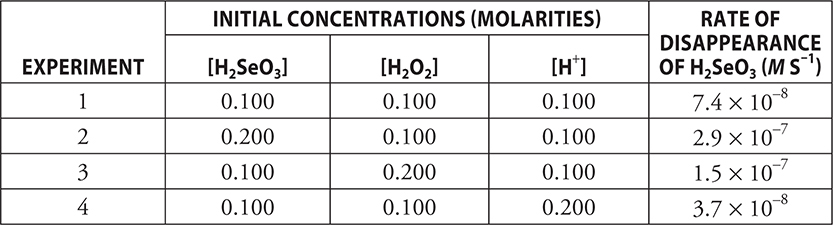
(a) Determine the order of the reaction for H2SeO3, H2O2, and H+. Justify your answers.
(b) Write the rate law for the reaction.
(c) Determine the value of the rate constant including units.
(d) (i) Is H2O2 acting as an oxidizing or as a reducing agent?
(ii) What is the oxidation state of oxygen in H2O2?
(iii) Write the balanced half-reaction for H2O2 in this reaction.
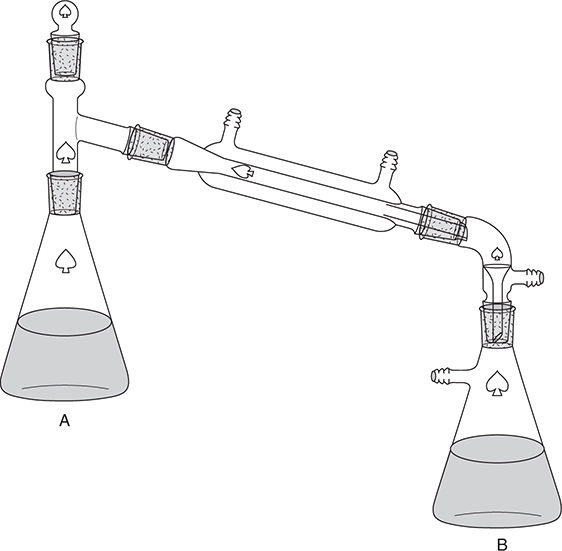
Question 3
A student wishes to analyze a sample of ammonium sulfate, (NH4)2SO4, contaminated with sodium sulfate, Na2SO4. She constructs the apparatus shown above to carry out this analysis. She weighs 1.002 grams of sample into flask A and dissolves the solid in 50 mL of water. Then she adds 50.00 mL of 0.4000 M hydrochloric acid, HCl, to flask B. In the next step, she quickly adds an excess of concentrated sodium hydroxide, NaOH, solution to flask A and quickly seals the system. She then heats flask A to boiling and distills over about 25 mL of water to flask B; during this process all the ammonia, NH3, generated in flask A transfers to flask B. After the distillation is complete, she disassembles the apparatus and adds a small amount of methyl red indicator to flask B and titrates the solution in flask B with standard sodium hydroxide solution. The titration requires 35.25 mL of 0.1600 M sodium hydroxide solution to reach the endpoint.
The reactions are:
(1) (NH4)2SO4(aq) + 2 NaOH(aq) → Na2SO4(aq) + 2 NH3(aq) + 2 H2O(l)
(2) NH3(aq) + HCl(aq) → NH4Cl(aq)
(3) NaOH(aq) + HCl(aq) → NaCl(aq) + H2O(l)
The first equation is for a weak acid (pKa NH4+ = 9.25)/strong base reaction. The second equation is for a weak base/strong acid reaction. The third equation is for a strong base/strong acid reaction.
Information on indicators:

(a) (i) Calculate the moles of hydrochloric acid originally in flask B.
(ii) Calculate the moles of hydrochloric acid reacting with the sodium hydroxide solution.
(iii) Calculate the moles of ammonia that reacted with the hydrochloric acid.
(b) (i) Calculate the mass of ammonium sulfate in the sample. (Molar mass of ammonium sulfate = 132.139 g)
(ii) Calculate the percent ammonium sulfate in the sample.
(c) (i) Strong acid strong base titrations commonly use phenolphthalein as an indicator. Give a good reason why the student chose to use methyl red instead.
(ii) A second student used phenolphthalein in place of methyl red and got significantly different results. Was the second student’s percent higher or lower than the student using methyl red? What was the cause of this discrepancy?
(d) In the box below, sketch the Lewis electron-dot structure of ammonium chloride.

Question 4
The following equipment is available for the determination of the molar mass of an unknown solid monoprotic acid, HA. Assume the unknown acid is a weak acid.

In addition to this equipment there is standardized hydrochloric acid, HCl, solution and unstandardized sodium hydroxide, NaOH, solution available and a suitable acid-base indicator such as phenolphthalein.
(a) Which of the above equipment is necessary for this experiment?
(b) Describe how the above equipment may be used to calculate the molar mass of the unknown solid.
(c) How would you calculate the Ka of the unknown acid?
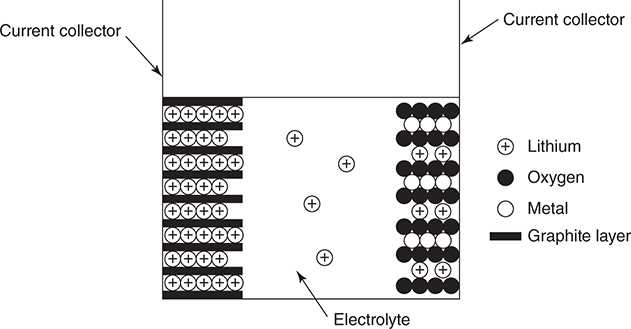
Question 5
The above schematic is one form of a lithium ion battery. The negative electrode (on the left) consists of graphite with lithium ions trapped between carbon layers. The positive electrode (on the right) is a layered metal oxide that allows lithium ions to enter the solid. The electrolyte is a lithium salt dissolved in an organic solvent. The negative electrode has the approximate composition LiC6, and the positive electrode can reach a composition of LiCoO2 in a fully discharged battery. The half-reactions are:
xLiC6(s) → xLi+ + xe— + xC6
Li1—xCoO2 + xLi+ + xe— → LiCoO2
(a) What is the balanced net ionic equation for the reaction?
(b) Why is it necessary to use an organic solvent for the electrolyte and not water?
(c) What is the oxidation state of cobalt in LiCoO2? Write the electron configuration for this cobalt ion in the ground state.
Question 6
Acetylene, C2H2, will react with a limited amount of bromine vapor, Br2, to form 1,2-dibromoethene, C2H2Br2. The product of the reaction is contaminated with unreacted acetylene and 1,1,2,2-tetrabromoethane, C2H2Br4. The key reactions are:
C2H2(g) + Br2(g) → C2H2Br2(g)
C2H2(g) + 2 Br2(g) → C2H2Br4(g)
(a) The left box below shows the Lewis electron-dot diagram for acetylene. The box on the right has an incomplete Lewis electron-dot diagram for 1,2-dibromoethene. Complete the diagram on the right by adding all the electron pairs.
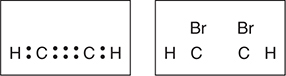
(b) In the box below, draw the complete Lewis electron-dot structure for 1,1,2,2-tetrabromoethane.

(c) Arrange the compounds C2H2, C2H2Br2, and C2H2Br4 in order of decreasing H—C—C bond angle. Why did you place these compounds in the order you predicted? Your explanation should include approximate bond angles.
Question 7
A student in Denver, Colorado, used the following reaction to generate oxygen gas in a gas generator:
2 KClO3(s) → 2 KCl(s) + 3 O2(g)
The gas generator was connected by a tube to an inverted flask, filled with water, in a water bath. The gas displaced all the water. The volume of the sample was 500.0 mL at 26°C, and the pressure in the room was 626 mm Hg. The vapor pressure of water at 26°C is 25 mm Hg.
A second student conducted a similar experiment under identical conditions beginning with the following reaction:
Mg3N2(s) + 6 H2O(l) → 3 Mg(OH)2(s) + 2 NH3(g)
The second student’s results were dramatically different from those of the first student (not as good), indicating that there was something drastically different.
(a) How many grams of oxygen are in the flask? Show all calculations.
(b) How many hydrogen atoms are in the flask after it is filled with oxygen?
(c) What is the most likely reason why the second student did not get good results? Explain your reasoning.
STOP. End of AP Chemistry Practice Exam 1, Section II (Free Response).
![]() Answers and Explanations for Exam 1, Section II (Free Response)
Answers and Explanations for Exam 1, Section II (Free Response)
Question 1
(a) (i) Ni(IO3)2(s) ⇆ Ni2+(aq) + 2 IO3—(aq)
You get 1 point for this answer. The equilibrium arrow and the ionic charges must be present.
(ii) Ksp = [Ni2+] [IO3—]2
You get 1 point for this answer. The charges must be present and the iodate ion concentration must be squared.
(iii) Ksp = [Ni2+] [IO3—]2 = 1.4 × 10—8
Setting [Ni2+] = x and [IO3—] = 2x and inserting into the mass-action expression gives:
(x) (2x)2 = 4x3 = 1.4 × 10—8.
Solving for x gives x = 1.5 × 10—3, and [IO3—] = 2x = 3.0 × 10—3 M.
You get 1 point for the correct [IO3—].
(b) Copper(II) nitrate is soluble in water (obviously because you are given a solution), which separates into copper(II) ions and nitrate ions. The copper(II) ion concentration is 0.10 M and it is a common ion affecting the equilibrium.
Ksp = [Cu2+] [IO3—]2 = 7.4 × 10—8
Setting [Cu2+] = 0.10 + x and [IO3—] = 2x and inserting into the mass-action expression gives:
(0.10 + x) (2x)2 = 7.4 × 10—8. Assuming x is much smaller than 0.10 allows a simplification of the calculation to (0.10) (2x)2 = 0.4 x2 = 7.4 × 10—8.
Solving for x gives x = 4.3 × 10—4 M, which leads to [IO3—] = 2x = 8.6 × 10—4 M.
You get 1 point for the correct [IO3—].
(c) (i) Silver iodate is more soluble in nitric acid than in pure water.
You get 1 point for this answer.
(ii) Iodic acid is a weak acid (Ka = 0.16); therefore, according to Le Châtelier’s principle, the solubility equilibrium will be displaced to the right as iodate ion combines with hydrogen ions from the nitric acid to form unionized iodic acid. The reactions are: ![]() and
and ![]()
You get 1 point for this answer.
(d) If the two compounds have the same stoichiometry, the larger Ksp would generate the higher iodate ion concentration. However, this is not the case, so it is necessary to calculate the iodate ion concentration for each.
Ksp = [Ba2+] [IO3—]2 = 1.5 × 10—9
Setting [Ba2+] = x and [IO3—] = 2x, and inserting into the mass-action expression gives:
(x) (2x)2 = 4x3 = 1.5 × 10—9.
Solving for x gives x = 7.2 × 10—4, and [IO3—] = 2x = 1.4 × 10—3 M.
Ksp = [La3+] [IO3—]3 = 6.2 × 10—12
Setting [La3+] = x and [IO3—] = 3x, and inserting into the mass-action expression gives:
(x) (3x)3 = 27x4 = 6.2 × 10—12.
Solving for x gives x = 6.9 × 10—4, and [IO3—] = 3x = 2.0 × 10—3 M.
You get 1 point for doing the calculations and showing La(IO3)3 is more soluble and 1 additional point for the correct answers.
(e) It is easier to begin with the balanced molecular equation:
2 NaIO3(aq) + Zn(NO3)2(aq) → Zn(IO3)2(s) + 2 NaNO3(aq)
Converting the molecular equation to a complete ionic equation:
2 Na+(aq) + 2 NO3—(aq) + Zn2+(aq) + 2 NO3—(aq) → Zn(IO3)2(s) + 2 Na+(aq) + 2 NO3—(aq)
The ionic compounds NaIO3 and Zn(IO3)2 should be separated into their ions since you are supplied with solutions and the sodium nitrate is also shown in the ionic form since you are told it is soluble in water. There is a Ksp for Zn(IO3)2 given in the table; therefore, it does not separate into ions.
Removing the spectator ions (Na+ and NO3—) from the complete ionic equation leaves the net ionic equation:
Zn2+(aq) + 2 IO3—(aq) → Zn(IO3)2(s)
You get 1 point for this answer.
Total your points for the different parts. There is a maximum of 10 points possible. Subtract one point if all answers did not have the correct number of significant figures.
Question 2
(a) The order for H2SeO3 is 2. The order for H2O2 is 1. The order for H+ is —1.
If you get all three of these correct, you get 1 point.
Comparing experiments 2 and 1 (placing the larger over the smaller makes the calculations a little easier): The concentration of H2SeO3 is doubled, and the rate is quadrupled; 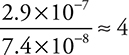 . This leads to H2SeO3 having an order of 2.
. This leads to H2SeO3 having an order of 2.
You get 1 point for this reasoning.
Comparing experiments 3 and 1: The concentration of H2O2 is doubled, and the rate is doubled; 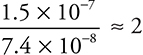 . This leads to H2O2 having an order of 1.
. This leads to H2O2 having an order of 1.
You get 1 point for this reasoning.
Comparing experiments 4 and 1: The concentration of H+ is doubled, and the rate is halved; 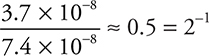 . This leads to H+ having an order of —1.
. This leads to H+ having an order of —1.
You get 1 point for this reasoning.
(b) Rate = k[H2SeO3]2[H2O2][H+]—1
This answer is worth 1 point. You will still get 1 point if you use incorrect orders from part (a).
(c) Rearrange the rate law to: 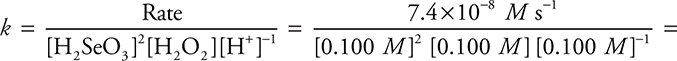 7.4 × 10—6 M—1 s—1
7.4 × 10—6 M—1 s—1
This example uses the values from experiment 1. You could use any of the four experiments.
You get 1 point for calculating the value of k. You get 1 additional point for the correct units.
(d) (i) H2O2 is acting as an oxidizing agent.
You get 1 point for this answer.
(ii) The oxidation state of oxygen in —1.
You get 1 point for this answer.
(iii) H2O2(aq) + 2 H+(aq) + 2 e— → 2 H2O(l)
You get 1 point for this answer.
Total your points for the different parts. There are 10 possible points. Subtract 1 point if you did not report the correct number of significant figures (2) in part (c).
Question 3
(a) (i) 50.00 mL 0.4000 M HCl
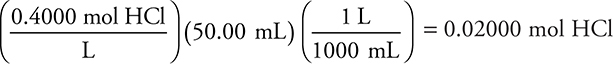 (4 significant figures)
(4 significant figures)
You get 1 point for this answer.
(ii) HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l)
35.25 mL 0.1600 M NaOH
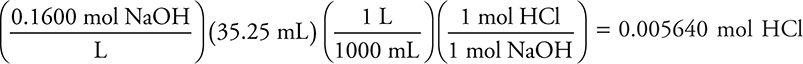 (4 significant figures)
(4 significant figures)
You get 1 point for this answer.
(iii) NH3(aq) + HCl(aq) → NH4Cl(aq)
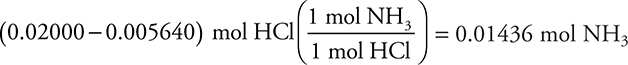 (4 significant figures)
(4 significant figures)
You get 1 point for this answer. If you miscalculated either of the preceding two answers but use your results correctly in this step, you still earn this point.
(b) (i) 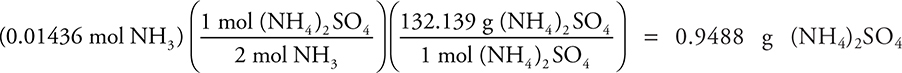 (4 significant figures)
(4 significant figures)
You get 1 point for this answer.
(ii) 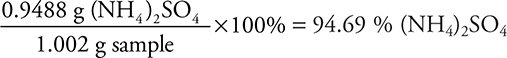 (4 significant figures)
(4 significant figures)
You get 1 point for this answer. If you got the wrong result for part (b)(i), but used it correctly here, you still get the point.
(c) (i) The sodium hydroxide solution will react with not only the hydrochloric acid (reaction 3) but also the ammonium ion (reaction 1). The methyl red endpoint is below where the ammonium ion begins to react; therefore, it is a better measure of the amount of HCl reacting than phenolphthalein, which has an endpoint after at least some of the ammonium ion has reacted.
You get 1 point for this answer.
(ii) The percent will be lower. It will be necessary to use more sodium hydroxide solution to reach the endpoint, making it appear that less ammonia reacted with the HCl. If there were less ammonia, then the percentage would be lower.
You get 1 point for predicting the percent will be lower and 1 point for the explanation.
(d)
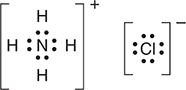
You get 1 point for having an octet on the nitrogen and an octet on the chlorine with no additional electrons shown.
You get 1 point for showing an ionic structure with no hint of a covalent bond between the ammonium ion and the chloride ion. The brackets are not essential but are present only as an aid to stress that these are ions with no covalent (electron-sharing).
Total your points. There is a maximum of 10 possible points. Subtract 1 point if any of your answers does not have the correct number of significant figures.
Question 4
(a) Analytical balance, buret, pipette, support stand and clamp, beakers or flask
You must have these five items to get 1 point.
(b) 1. It is necessary to standardize the sodium hydroxide solution.
(i) Pipette a known volume of the standardized hydrochloric acid into a beaker or flask (record the volume).
(ii) Add a small quantity of indicator to the acid.
(iii) Fill the buret with sodium hydroxide solution and take and record the initial volume.
(iv) Carefully add sodium hydroxide solution from the buret until the indicator indicates the endpoint.
(v) Record the final volume of sodium hydroxide solution in the buret.
(vi) The difference in the initial volume and final volume from the buret gives the quantity of sodium hydroxide solution added.
(vii) Use the recorded data and the concentration of the standard acid to determine the concentration of the sodium hydroxide solution.
The above steps are worth 1 point.
2. It is possible to determine the molar mass of the unknown acid using the standard sodium hydroxide solution.
(i) Weigh and record the mass of a sample of the unknown acid on an analytical balance.
(ii) Dissolve the unknown in a small quantity of DI water.
(iii) Fill the buret with sodium hydroxide solution and record the initial volume.
(iv) Carefully add sodium hydroxide solution from the buret until the indicator indicates the endpoint.
(v) Record the final volume of sodium hydroxide solution in the buret.
(vi) The difference in the initial volume and final volume from the buret gives the quantity of sodium hydroxide solution added.
(vii) Use the recorded data and the concentration of the sodium hydroxide solution to determine the moles of acid.
(viii) The mass of the acid divided by the moles of acid gives the molar mass of the acid.
The above steps are worth 1 point.
(c) (i) Weigh a small quantity of unknown acid into a beaker and add sufficient water to dissolve the solid.
(ii) Using the mass of the acid and the molar mass calculated above determine the volume of standard sodium hydroxide solution necessary to neutralize one-half of the acid in the beaker.
(iii) Fill the buret with sodium hydroxide solution and record the initial volume.
(iv) Carefully add sodium hydroxide solution from the buret until the calculated volume has been added.
(v) Measure the pH of the half-titrated solution with the pH meter provided.
(vi) The pH of the half-titrated solution is equal to the pKa of the acid and Ka = 10—pK.
The above steps are worth 1 point.
Total your points. There are 4 possible points.
Question 5
(a) Regardless of the value of x, it is the same in each half-reaction; therefore it is only necessary to add the two half-reactions together.
xLiC6(s) → xLi+ + xe— + xC6
Li1—xCoO2 + xLi+ + xe— → LiCoO2
Li1—xCoO2 + xLiC6(s) → LiCoO2 + xC6
You get 1 point for the correct equation.
(b) Lithium metal will react with water.
You get 1 point for this explanation.
(c) The oxidation state of cobalt is +3. This is determined by assuming the oxidation states of lithium and oxygen are +1 and —2, respectively. This leads to Li + Co + 2 (O) = 0 or (+1) + Co + 2 (—2) = 0.
The ground state electron configuration of Co3+ is 1s22s22p63s23p63d6 or [Ar]3d6.
You get 1 point for the correct oxidation state of cobalt and 1 point for the correct electron configuration. If you did not get the correct oxidation state, you can still get the second point if you give the correct electron configuration of the ion predicted to be present. Note that no reasonable ion will have any 4s electrons present.
Total your points; there are 4 points possible.
Question 6
(a)
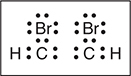
You get 1 point for the correct Lewis structure.
(b)
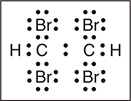
You get 1 point for the correct Lewis structure.
(c) The order is C2H2 > C2H2Br2 > C2H2Br4.
The structure of acetylene shown in the problem indicates a linear molecule with a HCC bond angle of 180°. The correct Lewis electron-dot diagram for C2H2Br2 indicates a trigonal planar arrangement about each carbon atom with a HCC bond angle of 120°. The correct Lewis electron-dot diagram for C2H2Br4 indicates a tetrahedral arrangement about each carbon atom with a HCC bond angle of 109°.
You get 1 point if you list the compounds in the correct order, and you get 1 point for a correct explanation including the bond angles.
Total your points for the problem. There is a maximum of 4 possible points.
Question 7
(a) 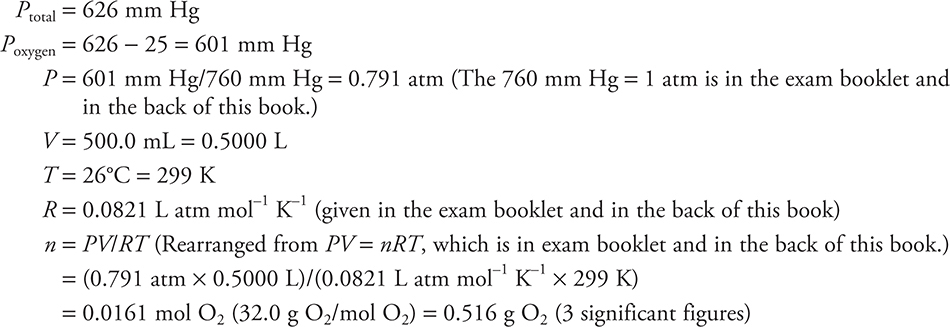
Give yourself 1 point for the correct answer (no deduction for rounding differently). You must include ALL parts of the calculation (including “=”). There is no deduction for combining steps. You cannot get this point if you did not subtract the vapor pressure of water from the total pressure.
(b) The hydrogen atoms are in the water molecules present in the flask; therefore it is necessary to calculate the mole of water present, convert to moles of hydrogen atoms, and use Avogadro’s number to convert from moles to the number of atoms.
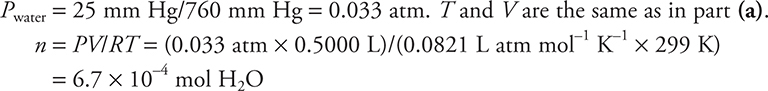
Number of H atoms = 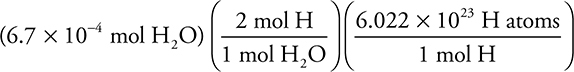 = 8.1 × 1020 H atoms
= 8.1 × 1020 H atoms
Give yourself 1 point for the correct answer (no deduction for rounding variations).
(c) Unlike oxygen gas, ammonia gas is very soluble in water because it, like water, is capable of hydrogen bonding. The formation of hydrogen between water molecules and ammonia molecules enhances the solubility of ammonia in water. Oxygen, on the other hand, is nonpolar and is only weakly attracted to water, giving oxygen a much lower solubility.
Give yourself 1 point for indicating the problem is the solubility of ammonia in water. Give yourself 1 additional point for the explanation if you invoke hydrogen bonding.
Total your points for this question. There are 4 points possible. Deduct 1 point if you reported the wrong number of significant figures in any answer.