Lippincott’s Illustrated Reviews: Biochemistr, Sixth Edition (2014)
UNIT II: Bioenergetics and Carbohydrate Metabolism
Chapter 10. Gluconeogenesis
I. OVERVIEW
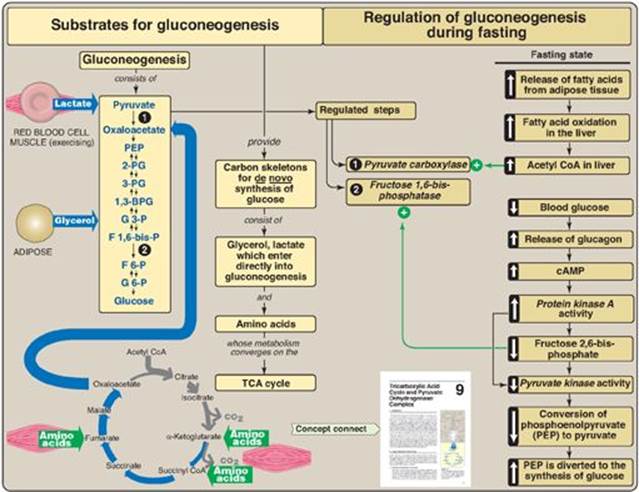
Some tissues, such as the brain, red blood cells (RBCs), kidney medulla, lens and cornea of the eye, testes, and exercising muscle, require a continuous supply of glucose as a metabolic fuel. Liver glycogen, an essential postprandial source of glucose, can meet these needs for only 10–18 hours in the absence of dietary intake of carbohydrate (see p. 329). During a prolonged fast, however, hepatic glycogen stores are depleted, and glucose is formed from noncarbohydrate precursors such as lactate, pyruvate, glycerol (derived from the backbone of triacylglycerols; see p. 190), and α-keto acids (derived from the catabolism of glucogenic amino acids; see p. 261). The formation of glucose does not occur by a simple reversal of glycolysis, because the overall equilibrium of glycolysis strongly favors pyruvate formation. Instead, glucose is synthesized by a special pathway, gluconeogenesis, which requires both mitochondrial and cytosolic enzymes. During an overnight fast, approximately 90% of gluconeogenesis occurs in the liver, with the remaining 10% occurring in the kidneys. However, during prolonged fasting, the kidneys become major glucose-producing organs, contributing an estimated 40% of the total glucose production. Figure 10.1 shows the relationship of gluconeogenesis to other essential pathways of energy metabolism.
Figure 10.1 The gluconeogenesis pathway shown as one of the essential pathways of energy metabolism. The numbered reactions are unique to gluconeogenesis. (See Figure 8.2, p. 92, for a more detailed map of metabolism.) P = phosphate.
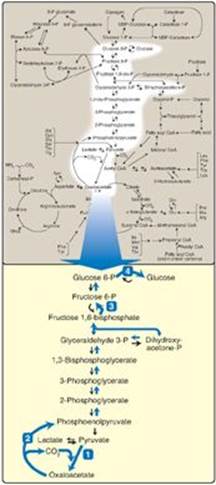
II. SUBSTRATES FOR GLUCONEOGENESIS
Gluconeogenic precursors are molecules that can be used to produce a net synthesis of glucose. The most important gluconeogenic prescurors are glycerol, lactate, and the α-keto acids obtained from the metabolism of glucogenic amino acids. [Note: Alanine, which directly gives rise to pyruvate, is an important example of a glucogenic amino acid.]
A. Glycerol
Glycerol is released during the hydrolysis of triacylglycerols in adipose tissue (see p. 190) and is delivered by the blood to the liver. Glycerol is phosphorylated by glycerol kinase to glycerol phosphate, which is oxidized by glycerol phosphate dehydrogenase to dihydroxyacetone phosphate, an intermediate of glycolysis. [Note: Adipocytes cannot phosphorylate glycerol because they essentially lack glycerol kinase.]
B. Lactate
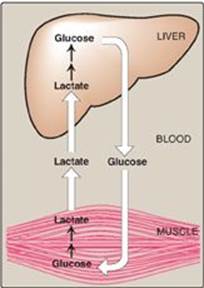
Lactate is released into the blood by exercising skeletal muscle and by cells that lack mitochondria such as RBCs. In the Cori cycle, bloodborne glucose is converted by exercising muscle to lactate, which diffuses into the blood. This lactate is taken up by the liver and reconverted to glucose, which is released back into the circulation (Figure 10.2).
C. Amino acids
Amino acids derived from hydrolysis of tissue proteins are the major sources of glucose during a fast. The metabolism of the glucogenic amino acids generates α-keto acids. α-Keto acids, such as α-ketoglutarate can enter the tricarboxylic acid (TCA) cycle and form oxaloacetate (OAA), a direct precursor of phosphoenolpyruvate (PEP). [Note: Acetyl coenzyme A (CoA) and compounds that give rise only to acetyl CoA (for example, acetoacetate and amino acids such as lysine and leucine) cannot give rise to a net synthesis of glucose. This is due to the irreversible nature of the pyruvate dehydrogenase (PDH) reaction, which converts pyruvate to acetyl CoA (see p. 109). These compounds give rise instead to ketone bodies (see p. 195) and are, therefore, termed ketogenic.]
Figure 10.2 The intertissue Cori cycle. [Note: Diffusion of lactate across membranes is facilitated by a transport protein.]
III. REACTIONS UNIQUE TO GLUCONEOGENESIS
Seven glycolytic reactions are reversible and are used in the synthesis of glucose from lactate or pyruvate. However, three of the reactions are irreversible and must be circumvented by four alternate reactions that energetically favor the synthesis of glucose. These reactions, unique to gluconeogenesis, are described below.
A. Carboxylation of pyruvate
The first “roadblock” to overcome in the synthesis of glucose from pyruvate is the irreversible conversion in glycolysis of PEP to pyruvate by pyruvate kinase (PK). In gluconeogenesis, pyruvate is first carboxylated by pyruvate carboxylase to OAA, which is then converted to PEP by the action of PEP-carboxykinase (Figure 10.3).
1. Biotin, a coenzyme: Pyruvate carboxylase requires biotin (see p. 381) covalently bound to the ε-amino group of a lysine residue in the enzyme (see Figure 10.3). Hydrolysis of ATP drives the formation of an enzyme–biotin–CO2 intermediate, which subsequently carboxylates pyruvate to form OAA. [Note: HCO3– is the source of the CO2.] The pyruvate carboxylase reaction occurs in the mitochondria of liver and kidney cells and has two purposes: to provide an important substrate for gluconeogenesis and to provide OAA that can replenish the TCA cycle intermediates that may become depleted, depending on the synthetic needs of the cell. Muscle cells also contain pyruvate carboxylase but use the OAA produced only for the replenishment (anaplerotic) purpose and do not synthesize glucose.
Pyruvate carboxylase is one of several carboxylases that require biotin. Others include acetyl CoA carboxylase (p. 183), propionyl CoA carboxylase (p. 193), and methylcrotonyl CoA carboxylase (p. 266).
2. Allosteric regulation: Pyruvate carboxylase is allosterically activated by acetyl CoA. Elevated levels of acetyl CoA in mitochondria signal a metabolic state in which the increased synthesis of OAA is required. For example, this occurs during fasting, when OAA is used for the synthesis of glucose by gluconeogenesis in the liver and kidney. Conversely, at low levels of acetyl CoA, pyruvate carboxylase is largely inactive, and pyruvate is primarily oxidized by the PDH complex to produce acetyl CoA that can be further oxidized by the TCA cycle (see p. 109).
Figure 10.3 Carboxylation of pyruvate to OAA, followed by reduction of OAA to malate for transfer to the cystol and subsequent decarboxylation to PEP. [Note: OAA can also be converted to PEP or aspartate for transfer to the cytosol.] MDm = mitochondrial malate dehydrogenase; MDc = cytosolic malate dehydrogenase.
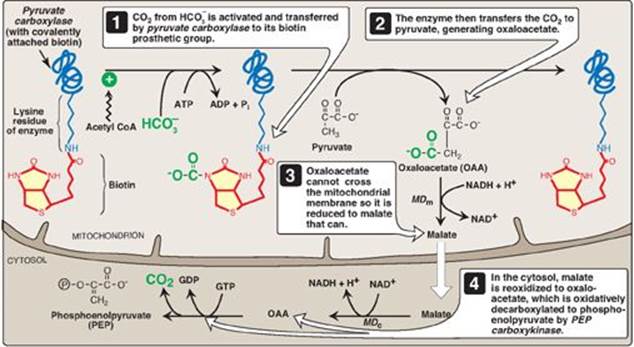
B. Transport of oxaloacetate to the cytosol
OAA must be converted to PEP for gluconeogenesis to continue. The enzyme that catalyzes this reaction is found in both the mitochondria and the cytosol in humans. The PEP generated in the mitochondria is transported to the cytosol by a specific transporter, whereas that generated in the cytosol requires the transport of OAA from the mitochondria to the cytosol. However, OAA is unable to be transported across the inner mitochondrial membrane, so it must first be reduced to malate by mitochondrial malate dehydrogenase (MD). Malate can be transported from the mitochondria to the cytosol, where it is reoxidized to OAA by cytosolic MD as nicotinamide adenine dinucleotide (NAD+) is reduced (see Figure 10.3). The NADH produced is used in the reduction of 1,3-bisphosphoglycerate to glyceraldehyde 3-phosphate (see p. 101), a step common to both glycolysis and gluconeogenesis. [Note: OAA also can be converted to aspartate, which is transported out of the mitochondria.]
C. Decarboxylation of cytosolic oxaloacetate
OAA is decarboxylated and phosphorylated to PEP in the cytosol by PEP-carboxykinase (also referred to as PEPCK). The reaction is driven by hydrolysis of guanosine triphosphate ([GTP] see Figure 10.3). The combined actions of pyruvate carboxylase and PEP-carboxykinase provide an energetically favorable pathway from pyruvate to PEP. PEP is then acted on by the reactions of glycolysis running in the reverse direction until it becomes fructose 1,6-bisphosphate.
The pairing of carboxylation with decarboxylation, as seen in gluconeogenesis, drives reactions that would otherwise be energetically unfavorable. A similar strategy is used in fatty acid synthesis (see pp. 183–184).
Figure 10.4 Dephosphorylation of fructose 1,6- bisphosphate. AMP = adenosine monophosphate; P = phosphate.
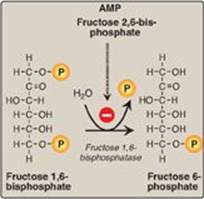
D. Dephosphorylation of fructose 1,6-bisphosphate
Hydrolysis of fructose 1,6-bisphosphate by fructose 1,6-bisphos-phatase, found in liver and kidney, bypasses the irreversible phosphofructokinase-1 (PFK-1) reaction, and provides an energetically favorable pathway for the formation of fructose 6-phosphate (Figure 10.4). This reaction is an important regulatory site of gluconeogenesis.
1. Regulation by energy levels within the cell: Fructose 1,6-bisphosphatase is inhibited by elevated levels of adenosine monophosphate (AMP), which signal an “energy-poor” state in the cell. Conversely, high levels of ATP and low concentrations of AMP stimulate gluconeogenesis, an energy-requiring pathway.
Figure 10.5 Effect of elevated glucagon on the intracellular concentration of fructose 2,6-bisphosphate in the liver. cAMP = cyclic AMP; PFK-2 = phosphofructokinase-2; FBP-2 = fructose 2,6-bisphosphatase; FBP-1 = fructose 1,6-bisphosphatase; P = phosphate.

2. Regulation by fructose 2,6-bisphosphate: Fructose 1,6-bisphos-phatase is inhibited by fructose 2,6-bisphosphate, an allosteric effector whose concentration is influenced by the insulin to glucagon ratio: when glucagon is high, the effector is not made and, thus, the phosphatase is active. (Figure 10.5). [Note: The signals that inhibit (low energy, high fructose 2,6-bisphosphate) or activate (high energy, low fructose 2,6-bisphosphate) gluconeogenesis have the opposite effect on glycolysis, providing reciprocal control of the pathways that synthesize and oxidize glucose (see p. 100).]
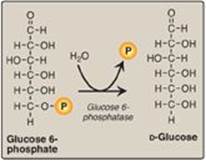
Figure 10.6 Dephosphorylation of glucose 6-phosphate allows release of free glucose from the liver and kidney into blood. P = phosphate.
E. Dephosphorylation of glucose 6-phosphate
Hydrolysis of glucose 6-phosphate by glucose 6-phosphatase bypasses the irreversible hexokinase/glucokinase reaction and provides an energetically favorable pathway for the formation of free glucose (Figure 10.6). Liver and kidney are the only organs that release free glucose from glucose 6-phosphate. This process actually requires a complex of two proteins: glucose 6-phosphate translocase, which transports glucose 6-phosphate across the endoplasmic reticular (ER) membrane, and the enzyme glucose 6-phosphatase (found only in gluconeogenic cells), which removes the phosphate, producing free glucose (see Figure 10.6). [Note: These ER-membrane proteins are also required for the final step of glycogen degradation (see p. 130). Type Ia and lb glycogen storage disease, caused by deficiencies in the phosphatase and the transferase, respectively, are characterized by severe fasting hypoglycemia, because free glucose is unable to be produced from either gluconeogenesis or glycogenolysis.] Specific glucose transporters (GLUTs) are responsible for moving free glucose into the cytosol and then into blood. [Note: Glucose 6-phosphate translocase moves inorganic phosphate out of the ER as it moves glucose 6-phosphate in.]
F. Summary of the reactions of glycolysis and gluconeogenesis
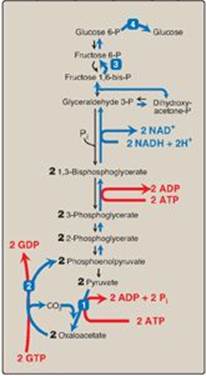
Of the 11 reactions required to convert pyruvate to free glucose, 7 are catalyzed by reversible glycolytic enzymes (Figure 10.7). The irreversible reactions of glycolysis catalyzed by hexokinase/glucokinase, PFK-1, and PK are circumvented by glucose 6-phosphatase, fructose 1,6-bisphosphatase, and pyruvate carboxylase/PEP-carboxykinase. In gluconeogenesis, the equilibria of the 7 reversible reactions of glycolysis are pushed in favor of glucose synthesis as a result of the essentially irreversible formation of PEP, fructose 6-phosphate, and glucose catalyzed by the gluconeogenic enzymes. [Note: The stoichiometry of gluconeogenesis from pyruvate couples the cleavage of six high-energy phosphate bonds and the oxidation of two NADH with the formation of each molecule of glucose (see Figure 10.7).]
Figure 10.7 Summary of the reactions of glycolysis and gluconeogenesis, showing the energy requirements of gluconeogenesis. The numbered reactions are unique to gluconeogenesis. P = phosphate; GDP = guanosine diphosphate; GTP = guanosine triphosphate; NAD(H) = nicotinamide adenine dinucleotide.
IV. REGULATION OF GLUCONEOGENESIS
The moment-to-moment regulation of gluconeogenesis is determined primarily by the circulating level of glucagon and by the availability of gluconeogenic substrates. In addition, slow adaptive changes in enzyme activity result from an alteration in the rate of enzyme synthesis or degradation or both. [Note: Hormonal control of the glucoregulatory system is presented in Chapter 23.]
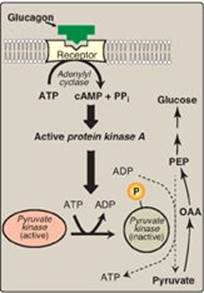
Figure 10.8 Covalent modification of pyruvate kinase results in inactivation of the enzyme. [Note: Only the hepatic isozyme is subject to covalent regulation.] OAA = oxaloacetate; PEP = phosphoenolpyruvate; cAMP = cyclic AMP; PPi = pyrophosphate; P = phosphate.
A. Glucagon
This peptide hormone from the a cells of pancreatic islets (see p. 313) stimulates gluconeogenesis by three mechanisms.
1. Changes in allosteric effectors: Glucagon lowers the level of fructose 2,6-bisphosphate, resulting in activation of fructose 1,6-bisphosphatase and inhibition of PFK-1, thus favoring gluconeogenesis over glycolysis (see Figure 10.5). [Note: See p. 99 for the role of fructose 2,6-bisphosphate in the regulation of glycolysis.]
2. Covalent modification of enzyme activity: Glucagon binds its G protein–coupled receptor (see p. 95) and, via an elevation in cyclic AMP (cAMP) level and cAMP-dependent protein kinase activity, stimulates the conversion of hepatic PK to its inactive (phosphorylated) form. This decreases the conversion of PEP to pyruvate, which has the effect of diverting PEP to the synthesis of glucose (Figure 10.8).
3. Induction of enzyme synthesis: Glucagon increases the transcription of the gene for PEP-carboxykinase, thereby increasing the availability of this enzyme as levels of its substrate rise during fasting. [Note: Glucocorticoids also increase expression of the gene, whereas insulin decreases expression.]
B. Substrate availability
The availability of gluconeogenic precursors, particularly glucogenic amino acids, significantly influences the rate of glucose synthesis. Decreased levels of insulin favor mobilization of amino acids from muscle protein and provide the carbon skeletons for gluconeogenesis. The ATP and NADH coenzymes-cosubstrates required for gluconeogenesis are primarily provided by the catabolism of fatty acids.
C. Allosteric activation by acetyl coenzyme A
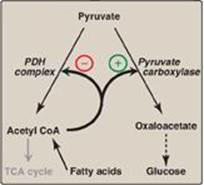
Allosteric activation of hepatic pyruvate carboxylase by acetyl CoA occurs during fasting. As a result of increased lipolysis in adipose tissue, the liver is flooded with fatty acids (see p. 330). The rate of formation of acetyl CoA by β-oxidation of these fatty acids exceeds the capacity of the liver to oxidize it to CO2 and H2O. As a result, acetyl CoA accumulates and activates pyruvate carboxylase. [Note: Acetyl CoA inhibits the PDH complex (by activating PDH kinase; see p. 111). Thus, this single compound can divert pyruvate toward gluconeogenesis and away from the TCA cycle (Figure 10.9).]
D. Allosteric inhibition by adenosine monophosphate
Fructose 1,6-bisphosphatase is inhibited by AMP—a compound that activates PFK-1. This results in a reciprocal regulation of glycolysis and gluconeogenesis seen previously with fructose 2,6-bisphosphate (see p. 121). [Note: Elevated AMP, thus, stimulates pathways that oxidize nutrients to provide energy for the cell.]
Figure 10.9 Acetyl coenzyme A (CoA) diverts pyruvate away from oxidation and toward gluconeogenesis. PDH = pyruvate dehydrogenase; TCA = tricarboxylic acid.
V. CHAPTER SUMMARY
Gluconeogenic precursors include the intermediates of glycolysis and the tricarboxylic acid cycle, glycerol released during the hydrolysis of triacylglycerols in adipose tissue, lactate released by cells that lack mitochondria and by exercising skeletal muscle, and α-keto acids derived from the metabolism of glucogenic amino acids (Figure 10.10). Seven of the reactions of glycolysis are reversible and are used for gluconeogenesis in the liver and kidneys. Three reactions are physiologically irreversible and must be circumvented. These reactions are catalyzed by the glycolytic enzymes pyruvate kinase, phosphofructokinase, and hexokinase. Pyruvate is converted to oxaloacetate and then to phosphoenolpyruvate (PEP) by pyruvate carboxylase and PEP-carboxykinase. The carboxylase requires biotin and ATP and is allosterically activated by acetyl coenzyme A. PEP-carboxykinaserequires GTP. The transcription of its gene is increased by glucagon and the glucocorticoids and decreased by insulin. Fructose 1,6-bisphosphate is converted to fructose 6-phosphate by fructose 1,6-bisphosphatase. This enzyme is inhibited by elevated levels of AMP and activated when ATP levels are elevated. The enzyme is also inhibited by fructose 2,6-bisphosphate, the primary allosteric activator of glycolysis. Glucose 6-phosphate is converted to glucose by glucose 6-phosphatase. This enzyme of the endoplasmic reticular membrane is required for the final step in gluconeogenesis as well as hepatic and renal glycogen degradation. Its deficiency results in severe, fasting hypoglycemia.
Figure 10.10 Key concept map for gluconeogenesis. TCA = tricarboxylic acid. CoA = coenzyme A; cAMP = cyclic adenosine monophosphate; P = phosphate; PG = phosphoglycerate; BPG = bisphosphoglycerate.
Study Questions
Choose the ONE best answer.
10.1 Which one of the following statements concerning gluconeogenesis is correct?
A. It is an energy-producing (exergonic) process.
B. It is important in maintaining blood glucose during a fast.
C. It is inhibited by a fall in the insulin-to-glucagon ratio.
D. It occurs in the cytosol of muscle cells.
E. It uses carbon skeletons provided by fatty acid degradation.
Correct answer = B. During a fast, glycogen stores are depleted, and gluconeogenesis maintains blood glucose. Gluconeogenesis is an energy-requiring (endergonic) pathway (both ATP and GTP get hydrolyzed) that occurs in liver, with kidney becoming a major glucose-producing organ in prolonged fasting. It utilizes both mitochondrial and cytosolic enzymes. Gluconeogenesis is stimulated by a fall in the insulin/glucagon ratio. Fatty acid degradation yields acetyl coenzyme A (CoA), which cannot be converted to glucose. This is because there is no net gain of carbons from acetyl CoA in the tricarboxylic acid cycle, and the pyruvate dehydrogenase reaction is physiologically irreversible. It is the carbon skeletons of most amino acids that are gluconeogenic.
10.2 Which reaction in the diagram below would be inhibited in the presence of large amounts of avidin, an egg white protein that binds and sequesters biotin?
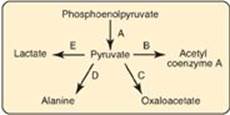
Correct answer = C. Pyruvate is carboxylated to oxaloacetate by pyruvate carboxylase, a biotin-requiring enzyme. B (PDH complex) requires thiamine pyrophosphate, lipoic acid, FAD, coenzyme A, NAD; D (transaminase) requires pyridoxal phosphate; E (lactate dehydrogenase) requires NADH.
10.3 Which one of the following reactions is unique to gluconeogenesis?
A. 1,3-Bisphosphoglycerate → 3-phosphoglycerate
B. Lactate → pyruvate
C. Oxaloacetate → phosphoenolpyruvate
D. Phosphoenolpyruvate → pyruvate
Correct answer = C. The other reactions are common to both gluconeogenesis and glycolysis.
10.4 Use the chart below to show the effect of adenosine monophosphate (AMP) and fructose 2,6-bisphosphate on the listed enzymes of gluconeogenesis and glycolysis.
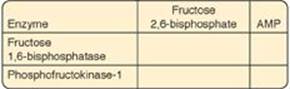
Both fructose 2,6-bisphosphate and adenosine monophosphate downregulate gluconeogenesis through inhibition of fructose 1,6-bisphosphatase and upregulate glycolysis through activation of phosphofructokinase-1. This results in reciprocal regulation of the two pathways.
10.5 The metabolism of ethanol by alcohol dehydrogenase produces reduced nicotinamide adenine dinucleotide (NADH). What effect is the change in the NAD+/NADH ratio expected to have on gluconeogenesis? Explain.
The increase in NADH as ethanol is oxidized will decrease the availability of oxaloacetate (OAA) because the reversible oxidation of malate to OAA by malate dehydrogenase of the tricarboxylic acid cycle is driven in the reverse direction by the high availability of NADH. Additionally, the reversible reduction of pyruvate to lactate by lactate dehydrogenase of glycolysis is driven in the forward direction by NADH. Thus, two important gluconeogenic substrates, OAA and pyruvate, are decreased as a result of the increase in NADH during ethanol metabolism. This results in a decrease in gluconeogenesis.
10.6 Given that acetyl coenzyme A cannot be a substrate for gluconeogenesis, why is its production in fatty acid oxidation essential for gluconeogenesis?
Acetyl coenzyme A inhibits the pyruvate dehydrogenase complex and activates pyruvate carboxylase, pushing pyruvate to gluconeogenesis and away from oxidation.