Lippincott’s Illustrated Reviews: Biochemistr, Sixth Edition (2014)
UNIT II: Bioenergetics and Carbohydrate Metabolism
Chapter 13. Pentose Phosphate Pathway and Nicotinamide Adenine Dinucleotide Phosphate
I. OVERVIEW
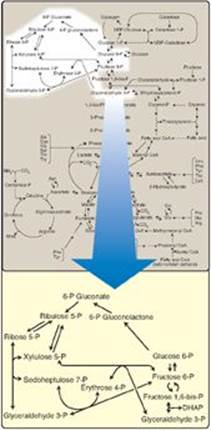
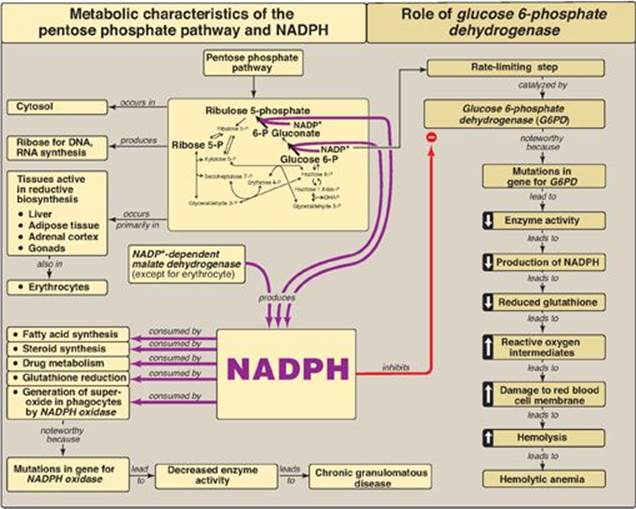
The pentose phosphate pathway (also called the hexose monophosphate shunt) occurs in the cytosol of the cell. It includes two irreversible oxidative reactions, followed by a series of reversible sugar–phosphate interconversions (Figure 13.1). No adenosine triphosphate (ATP) is directly consumed or produced in the cycle. Carbon 1 of glucose 6-phosphate is released as CO2, and two reduced nicotinamide adenine dinucleotide phosphates (NADPHs) are produced for each glucose 6-phosphate molecule entering the oxidative part of the pathway. The rate and direction of the reversible reactions of the pentose phosphate pathway are determined by the supply of and demand for intermediates of the cycle. The pathway provides a major portion of the body’s NADPH, which functions as a biochemical reductant. It also produces ribose 5-phosphate, required for the biosynthesis of nucleotides (see p. 293), and provides a mechanism for the metabolic use of five-carbon sugars obtained from the diet or the degradation of structural carbohydrates.
Figure 13.1 Pentose phosphate pathway shown as a component of the metabolic map (see Figure 8.2, p. 92 for a more detailed view of the metabolic pathways). P = phosphate; DHAP = dihydroxyacetone phosphate.
II. IRREVERSIBLE OXIDATIVE REACTIONS
The oxidative portion of the pentose phosphate pathway consists of three reactions that lead to the formation of ribulose 5-phosphate, CO2, and two molecules of NADPH for each molecule of glucose 6-phosphate oxidized (Figure 13.2). This portion of the pathway is particularly important in the liver, lactating mammary glands, and adipose tissue, which are active in the NADPH-dependent biosynthesis of fatty acids (see p. 186); in the testes, ovaries, placenta, and adrenal cortex, which are active in the NADPH-dependent biosynthesis of steroid hormones (see p. 237); and in red blood cells (RBCs), which require NADPH to keep glutathione reduced (see p. 152).
A. Dehydrogenation of glucose 6-phosphate
Glucose 6-phosphate dehydrogenase (G6PD) catalyzes an irreversible oxidation of glucose 6-phosphate to 6-phosphogluconolactone in a reaction that is specific for oxidized NADP (NADP+) as the coenzyme. The pentose phosphate pathway is regulated primarily at the G6PD reaction. NADPH is a potent competitive inhibitor of the enzyme, and, under most metabolic conditions, the ratio of NADPH/NADP+ is sufficiently high to substantially inhibit enzyme activity. However, with increased demand for NADPH, the ratio of NADPH/NADP+ decreases, and flux through the cycle increases in response to the enhanced activity of G6PD. Insulin upregulates expression of the gene for G6PD, and flux through the pathway increases in the absorptive state (see p. 323).
B. Formation of ribulose 5-phosphate
6-Phosphogluconolactone is hydrolyzed by 6-phosphogluconolactone hydrolase. The reaction is irreversible and not rate limiting. The oxidative decarboxylation of the product, 6-phosphogluconate, is catalyzed by 6-phosphogluconate dehydrogenase. This irreversible reaction produces a pentose sugar–phosphate (ribulose 5-phosphate), CO2 (from carbon 1 of glucose), and a second molecule of NADPH (see Figure 13.2).
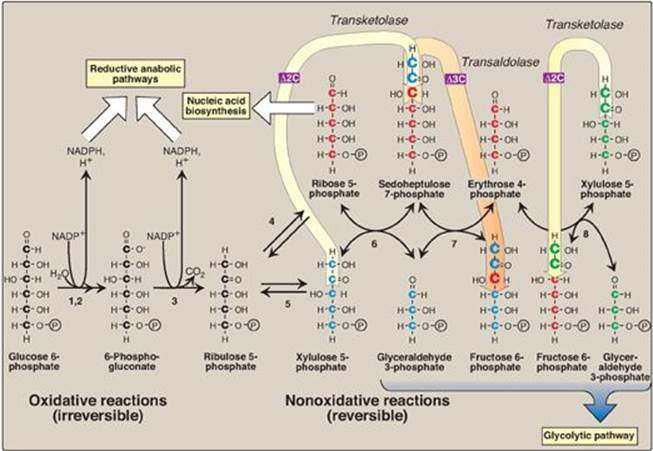
Figure 13.2 Reactions of the pentose phosphate pathway. Enzymes numbered above are: 1, 2) glucose 6-phosphate dehydrogenase and 6-phosphogluconolactone hydrolase, 3) 6-phosphogluconate dehydrogenase, 4) ribose 5-phosphate isomerase, 5) phosphopentose epimerase, 6 and 8) transketolase (coenzyme: thiamine pyrophosphate), and 7) transaldolase. ![]() = two carbons are transferred in transketolase reactions;
= two carbons are transferred in transketolase reactions; ![]() = three carbons are transferred in the transaldolase reaction. This can be represented as: 5C sugar + 5C sugar
= three carbons are transferred in the transaldolase reaction. This can be represented as: 5C sugar + 5C sugar ![]() 7C sugar + 3C sugar
7C sugar + 3C sugar ![]() 4C sugar + 6C sugar. NADP(H) = nicotinamide adenine dinucleotide phosphate; P = phosphate.
4C sugar + 6C sugar. NADP(H) = nicotinamide adenine dinucleotide phosphate; P = phosphate.
III. REVERSIBLE NONOXIDATIVE REACTIONS
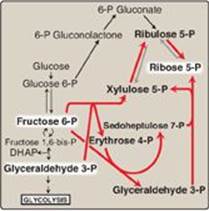
The nonoxidative reactions of the pentose phosphate pathway occur in all cell types synthesizing nucleotides and nucleic acids. These reactions catalyze the interconversion of sugars containing three to seven carbons (see Figure 13.2). These reversible reactions permit ribulose 5-phosphate (produced by the oxidative portion of the pathway) to be converted either to ribose 5-phosphate (needed for nucleotide synthesis; see p. 293) or to intermediates of glycolysis (that is, fructose 6-phosphate and glyceraldehyde 3-phosphate). For example, many cells that carry out reductive biosynthetic reactions have a greater need for NADPH than for ribose 5-phosphate. In this case, transketolase (which transfers two-carbon units in a thiamine pyrophosphate [TPP]-requiring reaction)and transaldolase (which transfers three-carbon units) convert the ribulose 5-phosphate produced as an end product of the oxidative reactions to glyceraldehyde 3-phosphate and fructose 6-phosphate, which are glycolytic intermediates. In contrast, when the demand for ribose for nucleotides and nucleic acids is greater than the need for NADPH, the nonoxidative reactions can provide the ribose 5-phosphate from glyceraldehyde 3-phosphate and fructose 6-phosphate in the absence of the oxidative steps (Figure 13.3).
In addition to transketolases, TPP is required by the enzyme complexes pyruvate dehydrogenase (see p. 110), α-ketoglutarate dehydrogenase of the citric acid cycle (see p. 112), and branched-chain α-keto acid dehydrogenase of branched-chain amino acid catabolism (see p. 266).
Figure 13.3 Formation of ribose 5-phosphate from intermediates of glycolysis. P = phosphate; DHAP = dihydroxyacetone phosphate.
IV. USES OF NADPH
The coenzyme NADPH differs from nicotinamide adenine dinucleotide (NADH) only by the presence of a phosphate group on one of the ribose units (Figure 13.4). This seemingly small change in structure allows NADPH to interact with NADPH-specific enzymes that have unique roles in the cell. For example, in the cytosol of hepatocytes the steady-state ratio of NADP+/NADPH is approximately 0.1, which favors the use of NADPH in reductive biosynthetic reactions. This contrasts with the high ratio of NAD+/NADH (approximately 1000), which favors an oxidative role for NAD+. This section summarizes some important NADP+ and NADPH-specific functions in reductive biosynthesis and detoxification reactions.
A. Reductive biosynthesis
NADPH can be thought of as a high-energy molecule, much in the same way as NADH. However, the electrons of NADPH are destined for use in reductive biosynthesis, rather than for transfer to oxygen as is the case with NADH (see p. 74). Thus, in the metabolic transformations of the pentose phosphate pathway, part of the energy of glucose 6-phosphate is conserved in NADPH, a molecule with a negative reduction potential (see p. 77), that, therefore, can be used in reactions requiring an electron donor, such as fatty acid (see p. 186) and steroid (see p. 237) synthesis.
Figure 13.4 Structure of reduced nicotinamide adenine dinucleotide phosphate (NADPH).
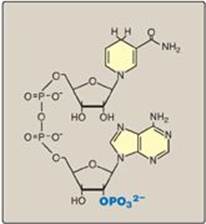
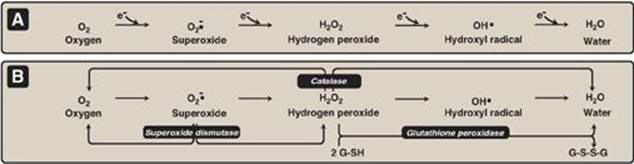
Figure 13.5 A. Formation of reactive intermediates from molecular oxygen. e- = electrons. B. Actions of antioxidant enzymes. G-SH = reduced glutathione; G-S-S-G = oxidized glutathione. (See Figure 13.6B for the regeneration of G-SH.)
B. Reduction of hydrogen peroxide
Hydrogen peroxide (H2O2) is one of a family of reactive oxygen species (ROS) that are formed from the partial reduction of molecular oxygen (Figure 13.5A). These compounds are formed continuously as byproducts of aerobic metabolism, through reactions with drugs and environmental toxins, or when the level of antioxidants is diminished, all creating the condition of oxidative stress. The highly reactive oxygen intermediates can cause serious chemical damage to DNA, proteins, and unsaturated lipids and can lead to cell death. ROS have been implicated in a number of pathologic processes, including reperfusion injury, cancer, inflammatory disease, and aging. The cell has several protective mechanisms that minimize the toxic potential of these compounds.
Figure 13.6 A. Structure of reduced glutathione (G-SH). [Note: Glutamate is linked to cysteine through a γ-carboxyl, rather than an α-carboxyl.] B. Glutathione-mediated reduction of hydrogen peroxide (H2O2) by reduced nicotinamide adenine dinucleotide phosphate (NADPH). G-S-S-G = oxidized glutathione.
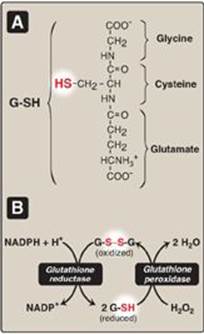
1. Enzymes that catalyze antioxidant reactions: Reduced glutathione (G-SH), a tripeptide-thiol (γ-glutamylcysteinylglycine) present in most cells, can chemically detoxify H2O2 (Figure 13.5B). This reaction, catalyzed by the selenium-containing glutathione peroxidase, forms oxidized glutathione (G-S-S-G), which no longer has protective properties. The cell regenerates G-SH in a reaction catalyzed by glutathione reductase, using NADPH as a source of reducing equivalents. Thus, NADPH indirectly provides electrons for the reduction of H2O2 (Figure 13.6). [Note: RBCs are totally dependent on the pentose phosphate pathway for their supply of NADPH because, unlike other cell types, RBCs do not have an alternate source for this essential coenzyme.] Additional enzymes, such as superoxide dismutase and catalase, catalyze the conversion of other reactive oxygen intermediates to harmless products (see Figure 13.5B). As a group, these enzymes serve as a defense system to guard against the toxic effects of ROS.
2. Antioxidant chemicals: A number of intracellular reducing agents, such as ascorbate (see p. 377), vitamin E (see p. 391), and β-carotene (see p. 382), are able to reduce and, thereby, detoxify reactive oxygen intermediates in the laboratory. Consumption of foods rich in these antioxidant compounds has been correlated with a reduced risk for certain types of cancers as well as decreased frequency of certain other chronic health problems. Therefore, it is tempting to speculate that the effects of these compounds are, in part, an expression of their ability to quench the toxic effect of ROS. However, clinical trials with antioxidants as dietary supplements have failed to show clear beneficial effects. In the case of dietary supplementation with β-carotene, the rate of lung cancer in smokers increased rather than decreased. Thus, the health-promoting effects of dietary fruits and vegetables likely reflect a complex interaction among many naturally occurring compounds, which has not been duplicated by consumption of isolated antioxidant compounds.
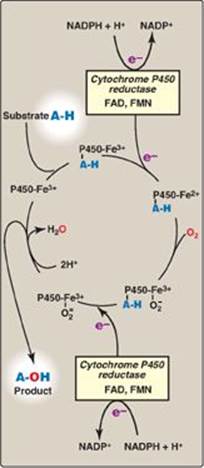
C. Cytochrome P450 monooxygenase system
Monooxygenases (mixed-function oxidases) incorporate one atom from molecular oxygen into a substrate (creating a hydroxyl group), with the other atom being reduced to water. In the cytochrome P450 monooxygenase system, NADPH provides the reducing equivalents required by this series of reactions (Figure 13.7). This system performs different functions in two separate locations in cells. The overall reaction catalyzed by a cytochrome P450enzyme is:
R-H + O2 + NADPH + H+ → R-OH + H2O + NADP+
where R may be a steroid, drug, or other chemical. [Note: Cyto-chrome P450 (CYP) enzymes are actually a superfamily of related, heme-containing monooxygenases that participate in a broad variety of reactions. The P450 in the name reflects the absorbance at 450 nm by the protein.]
1. Mitochondrial system: An important function of the cytochrome P450 monooxygenase system found associated with the inner mitochondrial membrane is the biosynthesis of steroid hormones. In steroidogenic tissues, such as the placenta, ovaries, testes, and adrenal cortex, it is used to hydroxylate intermediates in the conversion of cholesterol to steroid hormones, a process that makes these hydrophobic compounds more water soluble (see p. 237). The liver uses this same system in bile acid synthesis (see p. 224) and the hydroxylation of cholecalciferol to 25-hydroxycholecalciferol (vitamin D3; see p. 386), and the kidney uses it to hydroxylate vitamin D3 to its biologically active 1,25-dihydroxylated form.
2. Microsomal system: An extremely important function of the microsomal cytochrome P450 monooxygenase system found associated with the membrane of the smooth endoplasmic reticulum (particularly in the liver) is the detoxification of foreign compounds (xenobiotics). These include numerous drugs and such varied pollutants as petroleum products and pesticides. CYP enzymes of the microsomal system (for example, CYP3A4), can be used to hydroxylate these toxins. The purpose of these modifications is two-fold. First, it may itself activate or inactivate a drug and second, make a toxic compound more soluble, thereby facilitating its excretion in the urine or feces. Frequently, however, the new hydroxyl group will serve as a site for conjugation with a polar molecule, such as glucuronic acid (see p. 161), which will significantly increase the compound’s solubility. [Note: Polymorphisms (see p.473) in the genes for CYP enzymes can lead to differences in drug metabolism.]
Figure 13.7 Cytochrome P450 monooxygenase cycle (simplified). Electrons (e-) move from NADPH to FAD to FMN of the reductase and then to the heme iron (Fe) of the P450 enzyme. [Note: In the mitochondrial system, electrons move from FAD to an ironsulfur protein and then to the P450 enzyme.] FAD = flavin adenine dinucleotide; FMN = flavin mononucleotide; NADPH = reduced nicotinamide adenine dinucleotide phosphate.
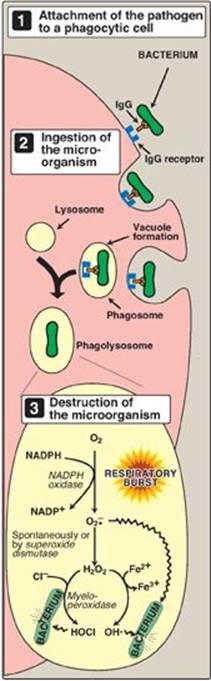
D. Phagocytosis by white blood cells
Phagocytosis is the ingestion by receptor-mediated endocytosis of microorganisms, foreign particles, and cellular debris by cells such as neutrophils and macrophages (monocytes). It is an important defense mechanism, particularly in bacterial infections. Neutrophils and monocytes are armed with both oxygen-independent and oxygen-dependent mechanisms for killing bacteria.
1. Oxygen-independent mechanism: Oxygen-independent mechanisms use pH changes in phagolysosomes and lysosomal enzymes to destroy pathogens.
2. Oxygen-dependent system: Oxygen-dependent mechanisms include the enzymes NADPH oxidase and myeloperoxidase (MPO) that work together in killing bacteria (Figure 13.8). Overall, the MPO system is the most potent of the bactericidal mechanisms. An invading bacterium is recognized by the immune system and attacked by antibodies that bind it to a receptor on a phagocytic cell. After internalization of the microorganism has occurred, NADPH oxidase, located in the leukocyte cell membrane, is activated and reduces O2 from the surrounding tissue to superoxide (![]() ), a free radical, as NADPH is oxidized. The rapid consumption of O2 that accompanies formation of
), a free radical, as NADPH is oxidized. The rapid consumption of O2 that accompanies formation of ![]() is referred to as the “respiratory burst.” [Note: Active NADPH oxidase is a membrane-associated complex containing a flavocytochrome plus additional peptides that translocate from the cytoplasm upon activation of the leukocyte. Electrons move from NADPH to O2 via flavin adenine nucleotide (FAD) and heme, generating
is referred to as the “respiratory burst.” [Note: Active NADPH oxidase is a membrane-associated complex containing a flavocytochrome plus additional peptides that translocate from the cytoplasm upon activation of the leukocyte. Electrons move from NADPH to O2 via flavin adenine nucleotide (FAD) and heme, generating ![]() . Rare genetic deficiencies in NADPH oxidase cause chronic granulomatous disease (CGD) characterized by severe, persistent infections and the formation of granulomas (nodular areas of inflammation) that sequester the bacteria that were not destroyed.] Next,
. Rare genetic deficiencies in NADPH oxidase cause chronic granulomatous disease (CGD) characterized by severe, persistent infections and the formation of granulomas (nodular areas of inflammation) that sequester the bacteria that were not destroyed.] Next, ![]() is converted to H2O2 (a ROS), either spontaneously or catalyzed by superoxide dismutase. In the presence of MPO, a heme-containing lysosomal enzyme present within the phagolysosome, peroxide plus chloride ions are converted to hypochlorous acid ([HOCl] the major component of household bleach), which kills the bacteria. The peroxide can also be partially reduced to the hydroxyl radical (OH•), a ROS, or be fully reduced to water by catalase or glutathione peroxidase. [Note: Deficiencies in MPO do not confer increased susceptibility to infection because peroxide from NADPH oxidase is bactericidal.]
is converted to H2O2 (a ROS), either spontaneously or catalyzed by superoxide dismutase. In the presence of MPO, a heme-containing lysosomal enzyme present within the phagolysosome, peroxide plus chloride ions are converted to hypochlorous acid ([HOCl] the major component of household bleach), which kills the bacteria. The peroxide can also be partially reduced to the hydroxyl radical (OH•), a ROS, or be fully reduced to water by catalase or glutathione peroxidase. [Note: Deficiencies in MPO do not confer increased susceptibility to infection because peroxide from NADPH oxidase is bactericidal.]
Figure 13.8 Phagocytosis and the oxygendependent pathway of microbial killing. IgG = the antibody immunoglobulin G; NADPH = reduced nicotinamide adenine dinucleotide phosphate; ![]() = superoxide; HOCl = hypochlorous acid; OH• = hydroxyl radical.
= superoxide; HOCl = hypochlorous acid; OH• = hydroxyl radical.
E. Synthesis of nitric oxide
Nitric oxide (NO) is recognized as a mediator in a broad array of biologic systems. NO is the endothelium-derived relaxing factor, which causes vasodilation by relaxing vascular smooth muscle. NO also acts as a neurotransmitter, prevents platelet aggregation, and plays an essential role in macrophage function. NO has a very short half-life in tissues (3–10 seconds) because it reacts with oxygen and superoxide and then is converted into nitrates and nitrites including peroxynitrite (O=NOO–), a reactive nitrogen species (RNS). [Note: NO is a free radical gas that is often confused with nitrous oxide (N2O), the “laughing gas” that is used as an anesthetic and is chemically stable.]
1. Nitric oxide synthase: Arginine, O2, and NADPH are substrates for cytosolic NO synthase ([NOS] Figure 13.9). Flavin mononucleotide (FMN), FAD, heme, and tetrahydrobiopterin (see p. 268) are coenzymes, and NO and citrulline are products of the reaction. Three NOS, each the product of a different gene, have been identified. Two are constitutive (synthesized at a constant rate), Ca2+–calmodulin-dependent enzymes (see p. 133). They are found primarily in endothelium (eNOS) and neural tissue (nNOS) and constantly produce very low levels of NO for vasodilation and neurotransmission. An inducible, Ca2+-independent enzyme (iNOS) can be expressed in many cells, including macrophages and neutrophils, as an early defense against pathogens. The specific inducers for iNOS vary with cell type, and include proinflammatory cytokines, such as tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ), and bacterial endotoxins such as lipopolysaccharide (LPS). These compounds promote synthesis of iNOS, which can result in large amounts of NO being produced over hours or even days.
2. Actions of nitric oxide on vascular endothelium: NO is an important mediator in the control of vascular smooth muscle tone. NO is synthesized by eNOS in endothelial cells and diffuses to vascular smooth muscle, where it activates the cytosolic form of guanylate cyclase (also known as guanylyl cyclase) to form cyclic guanosine monophosphate (cGMP). [Note: This reaction is analogous to the formation of cyclic AMP by adenylate cyclase (see p. 94), except that this guanylate cyclase is not membrane associated.] The resultant rise in cGMP causes activation of protein kinase G, which phosphorylates Ca2+ channels, causing decreased entry of Ca2+ into smooth muscle cells. This decreases the calcium–calmodulin activation of myosin light-chain kinase, thereby decreasing smooth muscle contraction and favoring relaxation. Vasodilator nitrates, such as nitroglycerin, are metabolized to NO, which causes relaxation of vascular smooth muscle and, therefore, lowers blood pressure. Thus, NO can be envisioned as an endogenous nitrovasodilator. [Note: NO is involved in penile erection. Sildenafil citrate, used in the treatment of erectile dysfunction, inhibits the phosphodiesterase that inactivates cGMP.]
3. Role of nitric oxide in macrophage bactericidal activity: In macrophages, iNOS activity is normally low, but synthesis of the enzyme is significantly stimulated by bacterial LPS and by release of IFN-γ and TNF-α in response to the infection. Activated macrophages form ![]() radicals (see p. 150) that combine with NO to form intermediates that decompose, producing the highly bactericidal OH• radical.
radicals (see p. 150) that combine with NO to form intermediates that decompose, producing the highly bactericidal OH• radical.
Figure 13.9 Synthesis and some of the actions of nitric oxide (NO). NADPH = reduced nicotinamide adenine dinucleotide phosphate. [Note: Flavin mononucleotide, flavin adenine dinucleotide, heme, and tetrahydrobiopterin are additional coenzymes required by NOS.]
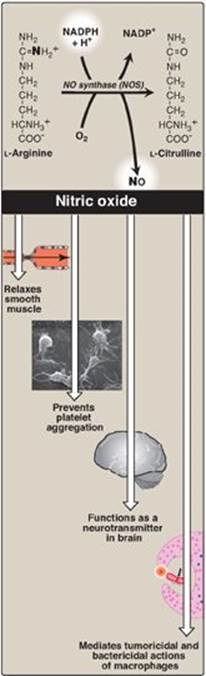
4. Other functions of nitric oxide: NO is a potent inhibitor of platelet adhesion and aggregation (by activating the cGMP pathway). It is also characterized as a neurotransmitter in the central and peripheral nervous systems.
Figure 13.10 Pathways of glucose 6-phosphate metabolism in the erythrocyte. NADP(H) = nicotinamide adenine dinucleotide phosphate; G-SH = reduced glutathionine; G-S-S-G = oxidized glutathionine; PPP = pentose phosphate pathway.
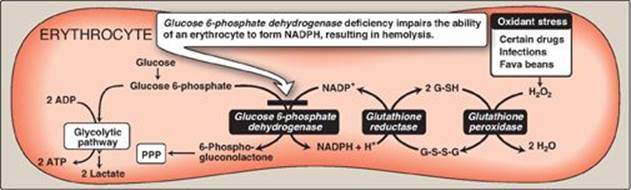
V. GLUCOSE 6-PHOSPHATE DEHYDROGENASE DEFICIENCY
G6PD deficiency is a hereditary disease characterized by hemolytic anemia caused by the inability to detoxify oxidizing agents. G6PD deficiency is the most common disease-producing enzyme abnormality in humans, affecting more than 400 million individuals worldwide. This deficiency has the highest prevalence in the Middle East, tropical Africa and Asia, and parts of the Mediterranean. G6PD deficiency is X linked and is, in fact, a family of deficiencies caused by a number of different mutations in the gene coding for G6PD. Only some of the resulting protein variants cause clinical symptoms. [Note: In addition to hemolytic anemia, a clinical manifestation of G6PDdeficiency is neonatal jaundice appearing 1–4 days after birth. The jaundice, which may be severe, typically results from increased production of unconjugated bilirubin (see p. 285).] The life span of individuals with a severe form of G6PD deficiency may be somewhat shortened as a result of complications arising from chronic hemolysis. This negative effect of G6PD deficiency has been balanced in evolution by an advantage in survival—an increased resistance to Plasmodium falciparum malaria. [Note: Sickle cell trait and β-thalassemia minor also confer resistance to malaria.]
A. Role of glucose 6-phosphate dehydrogenase in red blood cells
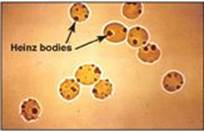
Diminished G6PD activity impairs the ability of the cell to form the NADPH that is essential for the maintenance of the G-SH pool. This results in a decrease in the cellular detoxification of free radicals and peroxides formed within the cell (Figure 13.10). G-SH also helps maintain the reduced states of sulfhydryl groups in proteins, including hemoglobin. Oxidation of those sulfhydryl groups leads to the formation of denatured proteins that form insoluble masses (called Heinz bodies) that attach to RBC membranes (Figure 13.11). Additional oxidation of membrane proteins causes RBCs to be rigid (less deformable), and they are removed from the circulation by macrophages in the spleen and liver. Although G6PD deficiency occurs in all cells of the affected individual, it is most severe in RBCs, where the pentose phosphate pathway provides the only means of generating NADPH. Other tissues have alternative sources for NADPH production (such as NADP+-dependent malate dehydrogenase [malic enzyme]; see p. 186) that can keep G-SH reduced. The RBC has no nucleus or ribosomes and cannot renew its supply of the enzyme. Thus, RBCs are particularly vulnerable to enzyme variants with diminished stability.
Figure 13.11 Heinz bodies in erythrocytes of a patient with glucose 6-phosphate dehydrogenase deficiency.
B. Precipitating factors in glucose 6-phosphate dehydrogenase deficiency
Most individuals who have inherited one of the G6PD mutations do not show clinical manifestations (that is, they are asymptomatic). However, some patients with G6PD deficiency develop hemolytic anemia if they are treated with an oxidant drug, ingest fava beans, or contract a severe infection.
1. Oxidant drugs: Commonly used drugs that produce hemolytic anemia in patients with G6PD deficiency are best remembered from the mnemonic AAA: antibiotics (for example, sulfamethoxazole and chloramphenicol), antimalarials (for example, primaquine but not chloroquine or quinine), and antipyretics (for example, acetanilid but not acetaminophen).
2. Favism: Some forms of G6PD deficiency, for example the Mediterranean variant, are particularly susceptible to the hemolytic effect of the fava (broad) bean, a dietary staple in the Mediterranean region. Favism, the hemolytic effect of ingesting fava beans, is not observed in all individuals with G6PD deficiency, but all patients with favism have G6PD deficiency.
3. Infection: Infection is the most common precipitating factor of hemolysis in G6PD deficiency. The inflammatory response to infection results in the generation of free radicals in macrophages, which can diffuse into the RBC and cause oxidative damage.
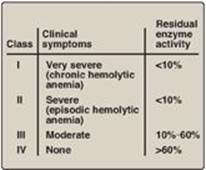
Figure 13.12 Classification of glucose 6-phosphate dehydrogenase (G6PD) deficiency variants. Note: Class V variants (not shown in table) result in overproduction of G6PD.
C. Properties of the variant enzymes
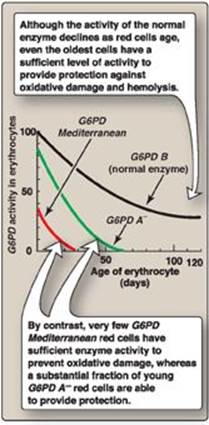
Almost all G6PD variants are caused by point mutations (see p. 433) in the gene for G6PD. Some mutations do not disrupt the structure of the enzyme’s active site and, therefore, do not affect enzymic activity. However, many mutant enzymes show altered kinetic properties. For example, variant enzymes may show decreased catalytic activity, decreased stability, or an alteration of binding affinity for NADP+, NADPH, or glucose 6-phosphate. The severity of the disease usually correlates with the amount of residual enzyme activity in the patient’s RBC. For example, variants can be classified as shown in Figure 13.12. G6PD A– is the prototype of the moderate (Class III) form of the disease. The RBCs contain an unstable but kinetically normal G6PD, with most of the enzyme activity present in the reticulocytes and younger RBCs (Figure 13.13). The oldest RBCs, therefore, have the lowest level of enzyme activity and are preferentially removed in a hemolytic episode. G6PD Mediterranean is the prototype of a more severe (Class II) deficiency in which the enzyme has decreased stability resulting in decreased enzymic activity. Class I mutations (rare) are the most severe and are associated with chronic nonspherocytic hemolytic anemia, which occurs even in the absence of oxidative stress.
Figure 13.13 Decline of erythrocyte glucose 6-phosphate dehydrogenase (G6PD) activity with cell age for the three most commonly encountered forms of the enzyme.
D. Molecular biology of glucose 6-phosphate dehydrogenase
The cloning of the gene for G6PD and the sequencing of its DNA (see p. 467) have permitted the identification of mutations that cause G6PD deficiency. More than 400 different G6PD variants have been identified, a finding that explains the numerous biochemical and clinical phenotypes that have been described. Most mutations that result in enzymic deficiency are missense mutations (see p. 433) in the coding region. Both G6PD A– and G6PDMediterranean represent mutant enzymes that differ from the respective normal variants by a single amino acid. Large deletions or frameshift mutations have not been identified, suggesting that complete absence of G6PDactivity is probably lethal.
VI. CHAPTER SUMMARY
The pentose phosphate pathway includes two irreversible oxidative reactions followed by a series of reversible sugar–phosphate interconversions (Figure 13.14). No ATP is directly consumed or produced in the cycle. The reduced nicotinamide adenine dinucleotide phosphate (NADPH)-producing oxidative portion of the pentose phosphate pathway is important in providing reducing equivalents for reductive biosynthesis and detoxification reactions. In this part of the pathway, glucose 6-phosphate is irreversibly converted to ribulose 5-phosphate, and two NADPH are produced. The regulated step is catalyzed by glucose 6-phosphate dehydrogenase (G6PD), which is strongly inhibited by NADPH. Reversible nonoxidative reactions interconvert sugars. This part of the pathway is the source of ribose 5-phosphate, required for nucleotide and nucleic acid synthesis. Because the reactions are reversible, they can be entered from fructose 6-phosphate and glyceraldehyde 3-phosphate (glycolytic intermediates) if ribose is needed and G6PD is inhibited. NADPH is a source of reducing equivalents in reductive biosynthesis, such as the production of fatty acids in liver, adipose tissue, and the mammary gland, and steroid hormones in the placenta, ovaries, testes, and adrenal cortex. It is also required by red blood cells (RBCs) for the reduction of hydrogen peroxide, providing the reducing equivalents required by glutathione (GSH). GSH is used by glutathione peroxidase to reduce peroxide to water. The oxidized glutathione (GSSH) produced is reduced by glutathione reductase, using NADPH as the source of electrons. NADPH provides reducing equivalents for the mitochondrial cytochrome P450 monooxygenase system, which is used in steroid hormone synthesis in steroidogenic tissue, bile acid synthesis in liver, and vitamin D activation in the liver and kidney. The microsomal system uses NADPH to detoxify foreign compounds (xenobiotics), such as drugs and a variety of pollutants. NADPH provides the reducing equivalents for phagocytes in the process of eliminating invading microorganisms. NADPH oxidase uses molecular oxygen and electrons from NADPH to produce superoxide radicals, which, in turn, can be converted to peroxide by superoxide dismutase. Myeloperoxidase catalyzes the formation of bactericidal hypochlorous acid from peroxide and chloride ions. Rare genetic defects in NADPH oxidase cause chronic granulomatous disease characterized by severe, persistent, infections and formation of granulomas. NADPH is required for the synthesis of nitric oxide (NO), an important free radical gas that causes vasodilation by relaxing vascular smooth muscle, acts as a neurotransmitter, prevents platelet aggregation, and helps mediate macrophage bactericidal activity. NO is made from arginine and O2 by three different NADPH-dependent NO synthases (NOS). The endothelial (eNOS), and neuronal (nNOS) isozymes constantly produce very low levels of NO for vasodilation and neurotransmission, respectively. The inducible isozyme (iNOS) produces large amounts of NO for defense against pathogens. G6PD deficiency impairs the ability of the cell to form the NADPH that is essential for the maintenance of the GSH pool. The cells most affected are the RBCsbecause they do not have additional sources of NADPH. G6PD deficiency is an X-linked disease characterized by hemolytic anemia caused by the production of free radicals and peroxides following administration of oxidant drugs, ingestion of fava beans, or severe infections. The extent of the anemia depends on the amount of residual enzyme. Class I variants, the most severe (and least common), are associated with chronic nonspherocytic hemolytic anemia. Babies with G6PD deficiency may experience neonatal jaundice.
Figure 13.14 Key concept map for the pentose phosphate pathway and nicotinamide adenine dinucleotide phosphate (NADPH).
Study Questions
Choose the ONE best answer.
13.1 In preparation for a trip to an area of India where chloroquine-resistant malaria is endemic, a young man is given primaquine prophylactically. Soon thereafter, he develops a hemolytic condition due to a deficiency in glucose 6-phosphate dehydrogenase. A less-than-normal level of which of the following is a consequence of the enzyme deficiency and the underlying cause of the hemolysis?
A. Glucose 6-phosphate
B. Oxidized form of nicotinamide adenine dinucleotide
C. Reduced form of glutathione
D. Ribose 5-phosphate
Correct answer = C. Glutathione (GSH) is essential for red cell integrity and is maintained in its reduced (functional) form by nicotinamide adenine dinucleotide phosphate (NADPH)-dependent glutathione reductase. The NADPH is generated by the oxidative portion of the pentose phosphate pathway. Individuals with a deficiency of the initiating and regulated enzyme of this pathway, glucose 6-phosphate dehydrogenase (G6PD), have a decreased ability to generate NADPH and, therefore, a decreased ability to keep GSH functional. When treated with an oxidant drug such as primaquine, some patients with G6PD deficiency develop a hemolytic anemia. Primaquine does not affect glucose 6-phosphate levels. Nicotinamide adenine dinucleotide is neither produced by the pentose phosphate pathway nor used as a coenzyme by GSH reductase.
13.2 Septic shock, a state of acute circulatory failure characterized by persistent arterial hypotension (low blood pressure) and inadequate organ perfusion refractory to fluid resuscitation, results from a severe inflammatory response to bacterial infection. It has a high mortality rate and is associated with changes in the level of nitric oxide. Which statement concerning septic shock is most likely correct?
A. Activation of endothelial nitric oxide synthase causes an increase in nitric oxide.
B. High mortality is the result of the long half-life of nitric oxide.
C. Lysine, the nitrogen source for nitric oxide synthesis, is deaminated by bacteria.
D. Overproduction of nitric oxide by a calcium-independent enzyme is the cause of the hypotension.
Correct answer = D. Overproduction of short-lived (not long-lived) nitric oxide (NO) by calcium-independent, inducible nitric oxide synthase (iNOS) results in excessive vasodilation leading to hypotension. NOS uses arginine, not lysine, as the source of the nitrogen. The endothelial enzyme (eNOS) is constitutive and produces low levels of NO at a consistent rate.
13.3 An individual who has recently been prescribed a drug (atorvastatin) to lower cholesterol levels is advised to limit consumption of grapefruit juice, because high intake of the juice reportedly results in an increased level of the drug in the blood, increasing the risk of side effects. Atorvastatin is a substrate for the cytochrome P450 enzyme CYP3A4, and grapefruit juice inhibits the enzyme. Which statement concerning P450 enzymes is most likely correct?
A. They accept electrons from reduced nicotinamide adenine dinucleotide (NADH).
B. They catalyze the hydroxylation of hydrophobic molecules.
C. They differ from nitric oxide synthase in that they contain heme.
D. They function in association with an oxidase.
Correct answer = B. The P450 enzymes hydroxylate hydrophobic compounds, making them more water soluble. Reduced nicotinamide adenine dinucleotide phosphate (NADPH) from the pentose phosphate pathway is the electron donor. The electrons are first transferred to the coenzymes of cytochrome P450 reductase and then to the P450 enzyme. Both the P450 enzymes and the nitric oxide synthase enzymes contain heme.
13.4 In male patients who are hemizygous for X-linked glucose 6-phosphate dehydrogenase deficiency, pathophysiologic consequences are more apparent in red blood cells (RBC) than in other cells such as in the liver. Which one of the following provides the most reasonable explanation for this different response?
A. Excess glucose 6-phosphate in the liver, but not in RBC, can be channeled to glycogen, thereby averting cellular damage.
B. Liver cells, in contrast to RBC, have alternative mechanisms for supplying the reduced nicotinamide adenine dinucleotide phosphate required for maintaining cell integrity.
C. Because RBC do not have mitochondria, production of ATP required to maintain cell integrity depends exclusively on the shunting of glucose 6-phosphate to the pentose phosphate pathway.
D. In RBC, in contrast to liver cells, glucose 6-phosphatase activity decreases the level of glucose 6-phosphate, resulting in cell damage.
Correct answer = B. Cellular damage is directly related to decreased ability of the cell to regenerate reduced glutathione, for which large amounts of reduced nicotinamide adenine dinucleotide phosphate (NADPH) are needed, and red blood cells (RBCs) have no means other than the pentose phosphate pathway of generating NADPH. It is decreased product (NADPH), not increased substrate (glucose 6-phosphate), that is the problem. RBCs do not have glucose 6-phosphatase. The pentose phosphate pathway does not generate ATP.
13.5 An essential prosthetic group for several enzymes of metabolism is derived from the vitamin thiamine. Measurement of the activity of what enzyme in red blood cells could be used to determine thiamine status in the body?
Red blood cells do not have mitochondria and, so, do not contain mitochondrial thiamine pyrophosphate (TPP)-requiring enzymes such as pyruvate dehydrogenase. However, they do contain the cytosolic TPP-requiring transketolase, whose activity can be used to assess thiamine status.