Lippincott’s Illustrated Reviews: Biochemistr, Sixth Edition (2014)
UNIT IV: Nitrogen Metabolism
Chapter 20. Amino Acid Degradation and Synthesis
I. OVERVIEW
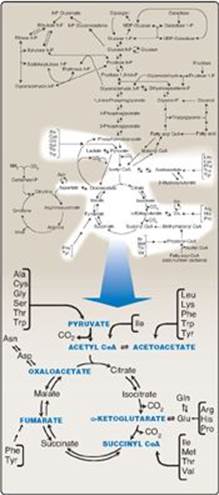
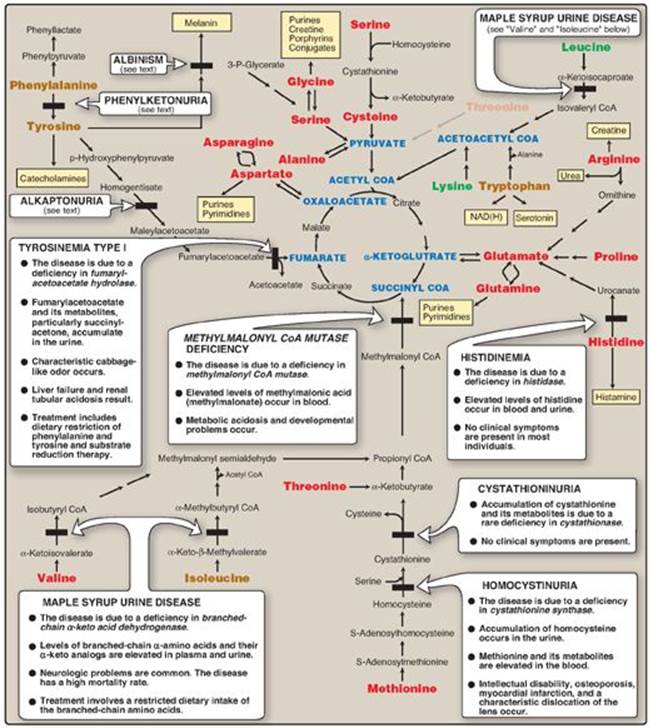
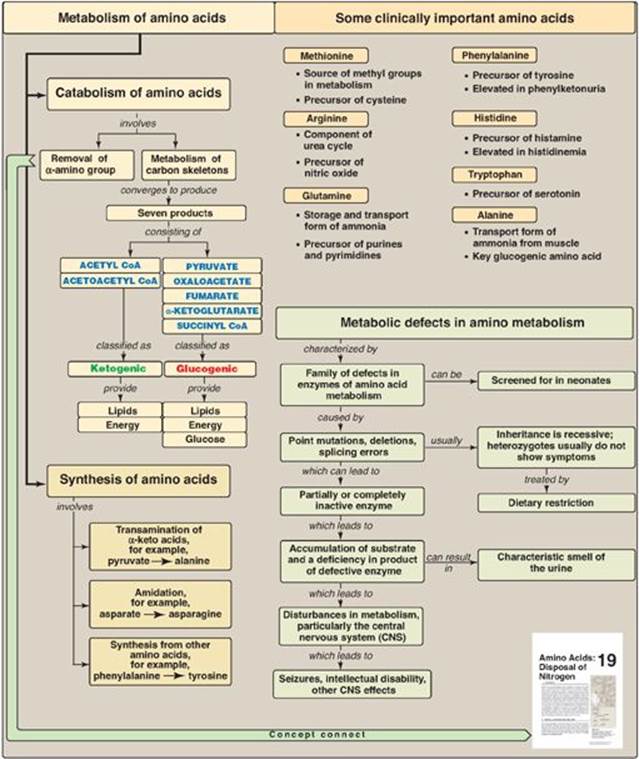
The catabolism of the amino acids involves the removal of α-amino groups, followed by the degradation of the resulting carbon skeletons. These pathways converge to form seven intermediate products: oxaloacetate, pyruvate, α-ketoglutarate, fumarate, succinyl coenzyme A (CoA), acetyl CoA, and acetoacetate. These products directly enter the pathways of intermediary metabolism, resulting either in the synthesis of glucose or lipid or in the production of energy through their oxidation to CO2 by the tricarboxylic acid (TCA) cycle. Figure 20.1 provides an overview of these pathways, with a more detailed summary presented in Figure 20.14 (see p. 269). Nonessential amino acids (Figure 20.2) can be synthesized in sufficient amounts from the intermediates of metabolism or, as in the case of cysteine and tyrosine, from essential amino acids. In contrast, the essential amino acids cannot be synthesized (or produced in sufficient amounts) by the body and, therefore, must be obtained from the diet in order for normal protein synthesis to occur. Genetic defects in the pathways of amino acid metabolism can cause serious disease.
Figure 20.1 Amino acid metabolism shown as a part of the essential pathways of energy metabolism. (See Figure 8.2, p. 92, for a more detailed view of these processes.) CoA = coenzyme A.
II. GLUCOGENIC AND KETOGENIC AMINO ACIDS
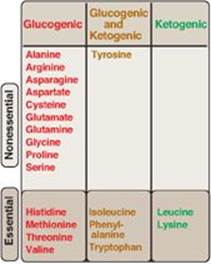
Amino acids can be classified as glucogenic, ketogenic, or both, based on which of the seven intermediates are produced during their catabolism (see Figure 20.2).
A. Glucogenic amino acids
Amino acids whose catabolism yields pyruvate or one of the intermediates of the TCA cycle are termed glucogenic. These intermediates are substrates for gluconeogenesis (see p. 117) and, therefore, can give rise to the net synthesis of glucose in the liver and kidney.

B. Ketogenic amino acids
Amino acids whose catabolism yields either acetoacetate or one of its precursors (acetyl CoA or acetoacetyl CoA) are termed ketogenic (see Figure 20.2). Acetoacetate is one of the ketone bodies, which also include 3-hydroxybutyrate and acetone (see p. 195). Leucine and lysine are the only exclusively ketogenic amino acids found in proteins. Their carbon skeletons are not substrates for gluconeogenesis and, therefore, cannot give rise to the net synthesis of glucose.
III. CATABOLISM OF THE CARBON SKELETONS OF AMINO ACIDS
The pathways by which amino acids are catabolized are conveniently organized according to which one (or more) of the seven intermediates listed above is produced from a particular amino acid.
A. Amino acids that form oxaloacetate
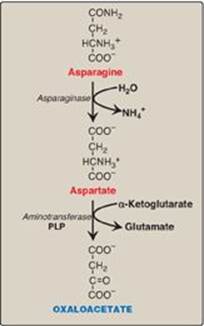
Asparagine is hydrolyzed by asparaginase, liberating ammonium (NH4+) and aspartate (Figure 20.3). Aspartate loses its amino group by transamination to form oxaloacetate (see Figure 20.3). [Note: Some rapidly dividing leukemic cells are unable to synthesize sufficient asparagine to support their growth. This makes asparagine an essential amino acid for these cells, which, therefore, require asparagine from the blood. Asparaginase, which hydrolyzes asparagine to aspartate, can be administered systemically to treat leukemic patients. Asparaginase lowers the level of asparagine in the plasma, thereby depriving cancer cells of a required nutrient.]
Figure 20.2 Classification of amino acids. [Note: Some amino acids can become conditionally essential. For example, supplementation with glutamine and arginine has been shown to improve outcomes in patients with trauma, postoperative infections, and immunosuppression.]
B. Amino acids that form α-ketoglutarate via glutamate
1. Glutamine: This amino acid is hydrolyzed to glutamate and ammonium by the enzyme glutaminase (see p. 256). Glutamate is converted to α-ketoglutarate by transamination or through oxidative deamination by glutamate dehydrogenase (see p. 252).
2. Proline: This amino acid is oxidized to glutamate. Glutamate is transaminated or oxidatively deaminated to form α-ketoglutarate.
3. Arginine: This amino acid is hydrolyzed by arginase to produce ornithine (and urea). [Note: This reaction occurs primarily in the liver as part of the urea cycle (see p. 255).] Ornithine is subsequently converted to α-ketoglutarate, with glutamate semialdehyde as an intermediate.
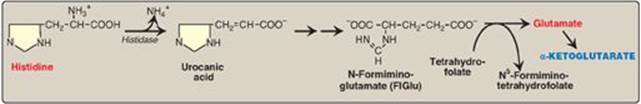
4. Histidine: This amino acid is oxidatively deaminated by histidase to urocanic acid, which subsequently forms N-formiminoglutamate ([FIGlu] Figure 20.4). FIGlu donates its formimino group to tetrahydrofolate (THF), leaving glutamate, which is degraded as described above. [Note: Individuals deficient in folic acid excrete increased amounts of FIGlu in the urine, particularly after ingestion of a large dose of histidine. The FIGlu excretion test has been used in diagnosing a deficiency of folic acid.] (See p. 267 for a discussion of folic acid, THF, and one-carbon metabolism.)
Figure 20.3 Metabolism of asparagine and aspartate. [Note: Recall that carbons from aspartate give rise to fumarate in the urea cycle (see p. 254).] PLP = pyridoxal phosphate.
C. Amino acids that form pyruvate
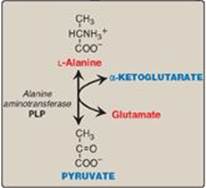
1. Alanine: This amino acid loses its amino group by transamination to form pyruvate (Figure 20.5). [Note: Alanine is the major gluconeogenic amino acid.]
Figure 20.4 Degradation of histidine.
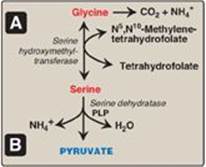
2. Serine: This amino acid can be converted to glycine and N5,N10-methylenetetrahydrofolate (Figure 20.6A). Serine can also be converted to pyruvate by serine dehydratase (Figure 20.6B).
3. Glycine: This amino acid can be converted to serine by the reversible addition of a methylene group from N5,N10-methylenetetrahydrofolic acid (see Figure 20.6A) or oxidized to CO2 and NH4+. [Note: Glycine can be deaminated to glyoxylate, which can be oxidized to oxalate or transaminated to glycine. Deficiency of the transaminase in liver peroxisomes causes overproduction of oxalate, the formation of oxalate stones, and kidney damage (primary oxaluria type 1).]
4. Cysteine: This amino acid undergoes desulfuration to yield pyruvate. [Note: The sulfate released can be used to synthesize 3-phosphoadenosine-5ʹ-phosphosulfate (PAPS), an activated sulfur donor to a variety of acceptors.] Cysteine can be oxidized to its disulfide derivative, cystine.
5. Threonine: This amino acid is converted to pyruvate in most organisms but is a minor pathway (at best) in humans.
Figure 20.5 Transamination of alanine to form pyruvate. PLP = pyridoxal phosphate.
D. Amino acids that form fumarate
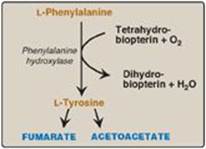
1. Phenylalanine and tyrosine: Hydroxylation of phenylalanine produces tyrosine (Figure 20.7). This reaction, catalyzed by tetrahydrobiopterin-requiring phenylalanine hydroxylase, initiates the catabolism of phenylalanine. Thus, the metabolism of phenylalanine and tyrosine merge, leading ultimately to the formation of fumarate and acetoacetate. Phenylalanine and tyrosine are, therefore, both glucogenic and ketogenic.
2. Inherited deficiencies: Inherited deficiencies in the enzymes of phenylalanine and tyrosine metabolism lead to the diseases phenylketonuria (see p. 270), tyrosimenia (see p. 269), and alkaptonuria (see p. 274) as well as the condition of albinism (see p. 273).
Figure 20.6 A. Interconversion of serine and glycine and oxidation of glycine. B. Dehydration of serine to form pyruvate. PLP = pyridoxal phosphate.
E. Amino acids that form succinyl coenzyme A: methionine
Methionine is one of four amino acids that form succinyl CoA. This sulfur-containing amino acid deserves special attention because it is converted to S-adenosylmethionine (SAM), the major methyl-group donor in one-carbon metabolism (Figure 20.8). Methionine is also the source of homocysteine, a metabolite associated with atherosclerotic vascular disease and thrombosis (see p. 265).
1. Synthesis of S-adenosylmethionine: Methionine condenses with adenosine triphosphate (ATP), forming SAM, a high-energy compound that is unusual in that it contains no phosphate. The formation of SAM is driven, in effect, by hydrolysis of all three phosphate bonds in ATP (see Figure 20.8).
Figure 20.7 Degradation of phenylalanine.
2. Activated methyl group: The methyl group attached to the tertiary sulfur in SAM is “activated” and can be transferred by methyltransferases to a variety of acceptor molecules such as norepinephrine in the synthesis of epinephrine (see p. 286). The methyl group is usually transferred to nitrogen (as with epinephrine) or oxygen atoms (as with the catechols; see p. 286) and sometimes to carbon atoms (as with cytosine). The reaction product, S-adenosylhomocysteine (SAH), is a simple thioether, analogous to methionine. The resulting loss of free energy makes methyl transfer essentially irreversible.
3. Hydrolysis of S-adenosylhomocysteine: After donation of the methyl group, SAH is hydrolyzed to homocysteine (Hcy) and adenosine. Hcy has two fates. If there is a deficiency of methionine, Hcy may be remethylated to methionine (see Figure 20.8). If methionine stores are adequate, Hcy may enter the transsulfuration pathway, where it is converted to cysteine.
a. Resynthesis of methionine: Hcy accepts a methyl group from N5-methyltetrahydrofolate (N5-methyl-THF) in a reaction requiring methylcobalamin, a coenzyme derived from vitamin B12 (see p. 375). [Note: The methyl group is transferred by methionine synthase from the B12 derivative to Hcy, regenerating methionine. Cobalamin is remethylated from N5-methyl-THF.]
b. Synthesis of cysteine: Hcy condenses with serine, forming cystathionine, which is hydrolyzed to α-ketobutyrate and cysteine (see Figure 20.8). This vitamin B6–requiring sequence has the net effect of converting serine to cysteine and Hcy to α-ketobutyrate, which is oxidatively decarboxylated to form propionyl CoA. Propionyl CoA is converted to succinyl CoA (see p. 194). Because Hcy is synthesized from the essential amino acid methionine, cysteine is not an essential amino acid as long as sufficient methionine is available.
Figure 20.8 Degradation and resynthesis of methionine. [Note: The resynthesis of methionine from homocysteine is the only reaction in which tetrahydrofolate both carries and donates a methyl (-CH3) group. In all other reactions, SAM is the methyl group carrier and donor.] PPi = pyrophosphate; Pi = inorganic phosphate.
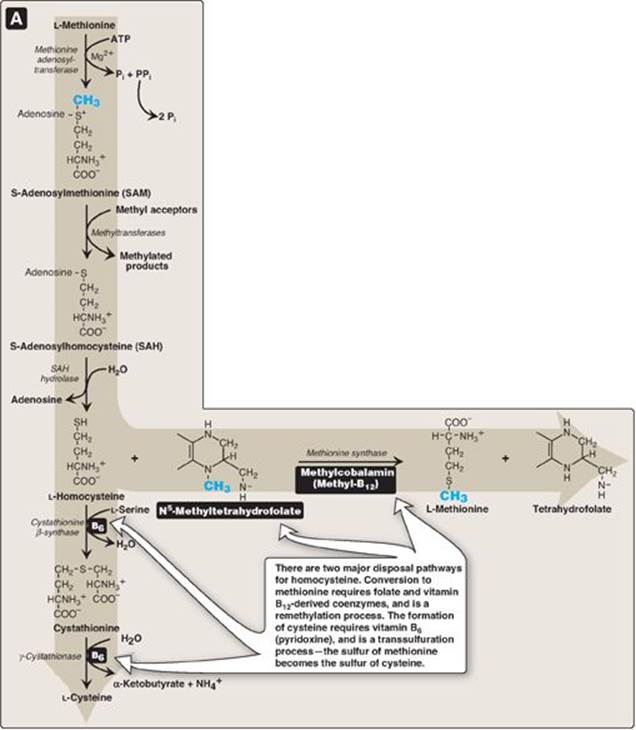
Figure 20.9 Association between cardiovascular disease mortality and total plasma homocysteine.
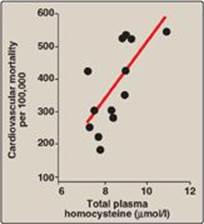
4. Relationship of homocysteine to vascular disease: Elevations in plasma Hcy levels promote oxidative damage, inflammation, and endothelial dysfunction and are an independent risk factor for occlusive vascular disease (Figure 20.9). Mild elevations are seen in about 7% of the population. Epidemiologic studies have shown that plasma Hcy levels are inversely related to plasma levels of folate, B12, and B6, the three vitamins involved in the conversion of Hcy to methionine or cysteine. Supplementation with these vitamins has been shown to reduce circulating levels of Hcy. However, in patients with established cardiovascular disease, vitamin therapy does not decrease cardiovascular events or death. This raises the question as to whether Hcy is a cause of the vascular damage or merely a marker of such damage. [Note: Large elevations in plasma Hcy as a result of rare deficiencies in cystathionine β-synthase of the transsulfuration pathway are seen in patients with classic homocystinuria. These individuals experience premature vascular disease, with about 25% dying from thrombotic complications before age 30 years.] Deficiencies in the remethylation reaction also result in a rise in Hcy.
Elevated homocysteine and decreased folic acid levels in pregnant women are associated with increased incidence of neural tube defects (improper closure, as in spina bifida) in the fetus. Periconceptual supplementation with folate reduces the risk of such defects.
F. Other amino acids that form succinyl coenzyme A
Degradation of valine, isoleucine, and threonine also results in the production of succinyl CoA, a TCA cycle intermediate and glucogenic compound.
1. Valine and isoleucine: These amino acids are branched-chain amino acids (BCAAs) that generate propionyl CoA, which is converted to methylmalonyl CoA and then succinyl CoA by biotin- and vitamin B12–requiring reactions (Figure 20.10).
2. Threonine: This amino acid is dehydrated to α-ketobutyrate, which is converted to propionyl CoA and then to succinyl CoA. Propionyl CoA, then, is generated by the catabolism of the amino acids methionine, valine, isoleucine, and threonine. [Note: Propionyl CoA also is generated by the oxidation of odd-numbered fatty acids (see p. 193).]
G. Amino acids that form acetyl coenzyme A or acetoacetyl coenzyme A
Leucine, isoleucine, lysine, and tryptophan form acetyl CoA or acetoacetyl CoA directly, without pyruvate serving as an intermediate (through the pyruvate dehydrogenase reaction; see p. 109). As noted earlier, phenylalanine and tyrosine also give rise to acetoacetate during their catabolism (see Figure 20.7). Therefore, there are a total of six partly or wholly ketogenic amino acids.
1. Leucine: This amino acid is exclusively ketogenic in its catabolism, forming acetyl CoA and acetoacetate (see Figure 20.10). The initial steps in the catabolism of leucine are similar to those of the other BCAAs, isoleucine and valine (see below).
2. Isoleucine: This amino acid is both ketogenic and glucogenic, because its metabolism yields acetyl CoA and propionyl CoA. The first three steps in the metabolism of isoleucine are similar to the initial steps in the degradation of the other BCAAs, valine and leucine (see Figure 20.10).
3. Lysine: This amino acid is exclusively ketogenic and is unusual in that neither of its amino groups undergoes transamination as the first step in catabolism. Lysine is ultimately converted to acetoacetyl CoA.
4. Tryptophan: This amino acid is both glucogenic and ketogenic because its catabolism yields alanine and acetoacetyl CoA. [Note: Quinolinate from tryptophan catabolism is used in the synthesis of nicotinamide adenine dinucleotide (NAD, see p. 379).]
Figure 20.10 Degradation of leucine, valine, and isoleucine. [Note: β-Methylcrotonyl CoA carboxylase is one of four biotinrequiring carboxylases we have encountered. The other three are pyruvate carboxylase, acetyl CoA carboxylase, and propionyl CoA carboxylase.] TPP = thiamine pyrophosphate; FAD = flavin adenine dinucleotide; CoA = coenzyme A; NAD = nicotinamide adenine dinucleotide; HMG = hydroxymethylglutarate.
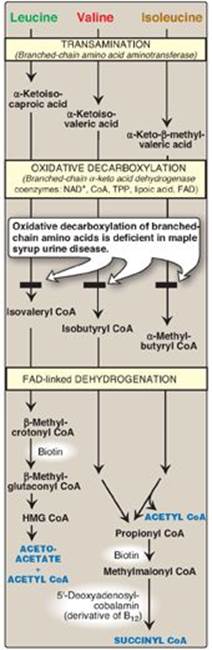
H. Catabolism of the branched-chain amino acids
The BCAAs isoleucine, leucine, and valine are essential amino acids. In contrast to other amino acids, they are metabolized primarily by the peripheral tissues (particularly muscle), rather than by the liver. Because these three amino acids have a similar route of catabolism, it is convenient to describe them as a group (see Figure 20.10).
1. Transamination: Transfer of the amino groups of all three BCAAs to α-ketoglutarate is catalyzed by a single, vitamin B6–requiring enzyme, branched-chain amino acid aminotransferase.
2. Oxidative decarboxylation: Removal of the carboxyl group of the α-keto acids derived from leucine, valine, and isoleucine is catalyzed by a single multienzyme complex, branched-chain α-keto acid dehydrogenase (BCKD) complex. This complex uses thiamine pyrophosphate, lipoic acid, flavin adenine dinucleotide (FAD), NAD+, and CoA as its coenzymes and produces NADH. [Note: This reaction is similar to the conversion of pyruvate to acetyl CoA by pyruvate dehydrogenase (PDH) complex (see p. 110) and the oxidation of α-ketoglutarate to succinyl CoA by α-ketoglutarate dehydrogenase complex (see p. 112). The Enzyme 3 (E3) component is identical in BCKD, PDH, and α-ketoglutarate dehydrogenase.]
3. Dehydrogenation: Oxidation of the products formed in the BCKD reaction yields α-β-unsaturated acyl CoA derivatives and FADH2. This reaction is analogous to the FAD-linked dehydrogenation in the β-oxidation of fatty acids (see p. 192). [Note: Deficiency in the dehydrogenase specific for isovaleryl CoA causes neurologic problems and is associated with a “sweaty feet” odor in body fluids.]
4. End products: The catabolism of isoleucine ultimately yields acetyl CoA and succinyl CoA, rendering it both ketogenic and glucogenic. Valine yields succinyl CoA and is glucogenic. Leucine is ketogenic, being metabolized to acetoacetate and acetyl CoA. In addition, NADH and FADH2 are produced in the decarboxylation and dehydrogenation reactions, respectively. [Note: BCAA catabolism also results in glutamine and alanine being sent out into the blood from muscle (see p. 253).]
IV. FOLIC ACID AND AMINO ACID METABOLISM
Some synthetic pathways require the addition of single carbon groups that exist in a variety of oxidation states, including formyl, methenyl, methylene, and methyl. These single carbon groups can be transferred from carrier compounds such as THF and SAM to specific structures that are being synthesized or modified. The “one-carbon pool” refers to the single carbon units attached to this group of carriers. [Note: CO2, the dehydrated form of carbonic acid, is carried by the vitamin biotin, which is a prosthetic group for most carboxylation reactions but is not considered a member of the one-carbon pool. Defects in the ability to add or remove biotin from carboxylases result in multiple carboxylase deficiency. Treatment is supplementation with biotin.]
A. Folic acid and one-carbon metabolism
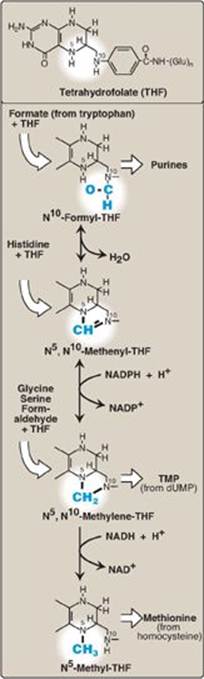
The active form of folic acid, THF, is produced from folate by dihydrofolate reductase in a two-step reaction requiring two nicotinamide adenine dinucleotide phosphates (NADPH). The one-carbon unit carried by THF is bound to nitrogen N5 or N10 or to both N5 and N10. Figure 20.11 shows the structures of the various members of the THF family and their interconversions and indicates the sources of the one-carbon units and the synthetic reactions in which the specific members participate. [Note: Folate deficiency presents as a megaloblastic anemia due to decreased availability of the purines and of the thymidine monophosphate needed for DNA synthesis (see p. 303).]
Figure 20.11 Summary of the interconversions and uses of the carrier tetrahydrofolate. [Note: N5, N10-Methenyl-THF also arises from N5-formimino-THF (see Figure 20.4).] NADP(H) = nicotinamide adenine dinucleotide phosphate; NAD(H) = nicotinamide adenine dinucleotide; TMP = thymidine monophosphate; dUMP = deoxyuridine monophosphate.
V. BIOSYNTHESIS OF NONESSENTIAL AMINO ACIDS
Nonessential amino acids are synthesized from intermediates of metabolism or, as in the case of tyrosine and cysteine, from the essential amino acids phenylalanine and methionine, respectively. The synthetic reactions for the nonessential amino acids are described below and are summarized later in Figure 20.14. [Note: Some amino acids found in proteins, such as hydroxyproline and hydroxylysine (see p. 45), are modified after their incorporation into the protein (posttranslational modification; see p. 443).]
A. Synthesis from α-keto acids
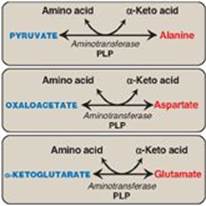
Alanine, aspartate, and glutamate are synthesized by transfer of an amino group to the α-keto acids pyruvate, oxaloacetate, and α-ketoglutarate, respectively. These transamination reactions (Figure 20.12; also see p. 250) are the most direct of the biosynthetic pathways. Glutamate is unusual in that it can also be synthesized by the reverse of oxidative deamination, catalyzed by glutamate dehydrogenase, when ammonia levels are high (see p. 252).
B. Synthesis by amidation
1. Glutamine: This amino acid, which contains an amide linkage with ammonia at the γ-carboxyl, is formed from glutamate by glutamine synthetase (see Figure 19.18, p. 256). The reaction is driven by the hydrolysis of ATP. In addition to producing glutamine for protein synthesis, the reaction also serves as a major mechanism for the transport of ammonia in a nontoxic form (see p. 256 for a discussion of ammonia metabolism).
2. Asparagine: This amino acid, which contains an amide linkage with ammonia at the β-carboxyl, is formed from aspartate by asparagine synthetase, using glutamine as the amide donor. Like the synthesis of glutamine, the reaction requires ATP and has an equilibrium far in the direction of amide synthesis.
Figure 20.12 Formation of alanine, aspartate, and glutamate from the corresponding α-keto acids. PLP = pyridoxal phosphate.
C. Proline
Glutamate via glutamate semialdehyde is converted to proline by cyclization and reduction reactions. [Note: The semialdehyde can also be transaminated to ornithine.]
D. Serine, glycine, and cysteine
1. Serine: This amino acid arises from 3-phosphoglycerate, an intermediate in glycolysis (see Figure 8.18, p. 101), which is first oxidized to 3-phosphopyruvate and then transaminated to 3-phosphoserine. Serine is formed by hydrolysis of the phosphate ester. Serine can also be formed from glycine through transfer of a hydroxymethyl group by serine hydroxymethyltransferase using N5,N10-methylene-THF as the one-carbon donor (see Figure 20.6A). [Note: Selenocysteine (Sec), the 21st genetically encoded amino acid, is synthesized from serine and selenium while serine is attached to transfer RNA. Sec is found in several proteins such as glutathione peroxidase(see p. 148).]
2. Glycine: This amino acid is synthesized from serine by removal of a hydroxymethyl group, also by serine hydroxymethyltransferase (see Figure 20.6A). THF is the one-carbon acceptor.
3. Cysteine: This amino acid is synthesized by two consecutive reactions in which Hcy combines with serine, forming cystathionine, which, in turn, is hydrolyzed to α-ketobutyrate and cysteine (see Figure 20.8). (Hcy is derived from methionine as described on p. 264.) Because methionine is an essential amino acid, cysteine synthesis can be sustained only if the dietary intake of methionine is adequate.
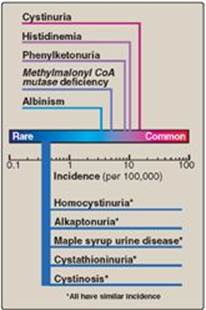
Figure 20.13 Incidence of inherited diseases of amino acid metabolism. [Note: Cystinuria is the most common genetic error of amino acid transport.]
E. Tyrosine
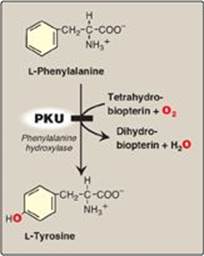
Tyrosine is formed from phenylalanine by phenylalanine hydroxylase. The reaction requires molecular oxygen and the coenzyme tetrahydrobiopterin (BH4), which is synthesized from guanosine triphosphate. One atom of molecular oxygen becomes the hydroxyl group of tyrosine, and the other atom is reduced to water. During the reaction, BH4 is oxidized to dihydrobiopterin (BH2). BH4 is regenerated from BH2 by NADH-requiring dihydropteridine reductase. Tyrosine, like cysteine, is formed from an essential amino acid and is, therefore, nonessential only in the presence of adequate dietary phenylalanine.
Figure 20.14 Summary of the metabolism of amino acids in humans. Genetically determined enzyme deficiencies are summarized in white boxes. Nitrogen-containing compounds derived from amino acids are shown in small, yellow boxes. Classification of amino acids is color coded: Red = glucogenic; brown = glucogenic and ketogenic; green = ketogenic. Compounds in BLUE ALL CAPS are the seven metabolites to which all amino acid metabolism converges. CoA = coenzyme A; NAD(H) = nicotinamide adenine dinucleotide.
VI. METABOLIC DEFECTS IN AMINO ACID METABOLISM
Inborn errors of metabolism are commonly caused by mutant genes that generally result in abnormal proteins, most often enzymes. The inherited defects may be expressed as a total loss of enzyme activity or, more frequently, as a partial deficiency in catalytic activity. Without treatment, the inherited defects of amino acid metabolism almost invariably result in intellectual disability or other developmental abnormalities as a consequence of harmful accumulation of metabolites. Although more than 50 of these disorders have been described, many are rare, occurring in less than 1 per 250,000 in most populations (Figure 20.13). Collectively, however, they constitute a very significant portion of pediatric genetic diseases (Figure 20.14). Phenylketonuria (PKU) is an important disease of amino acid metabolism because it is relatively common and responds to dietary treatment.
Figure 20.15 A deficiency in phenylalanine hydroxylase results in the disease phenylketonuria (PKU).
Screening of newborns for a number of treatable disorders, including those of amino acid metabolism, is done by tandem mass spectrometry of blood obtained from a heel prick. By law, all states must screen for over 20 disorders, with some screening for over 30. All states screen for PKU.
A. Hyperphenylalanemia and phenylketonuria
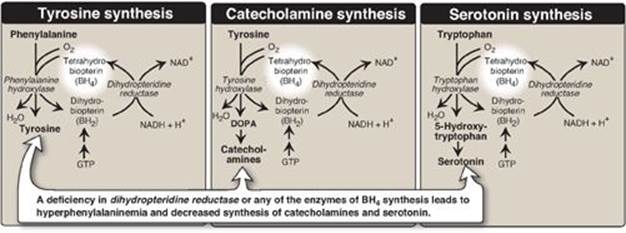
PKU, caused by a deficiency of phenylalanine hydroxylase ([PAH] Figure 20.15), is the most common clinically encountered inborn error of amino acid metabolism (incidence 1:15,000). Biochemically, it is characterized by accumulation of phenylalanine, resulting in hyperphenylalanemia, and a deficiency of tyrosine. It is treated by dietary restriction of phenylalanine. Hyperphenylalanemia may also be caused by deficiencies in any of the several enzymes required to synthesize BH4 or in dihydropteridine reductase, which regenerates BH4 from BH2 (Figure 20.16). Such deficiencies indirectly raise phenylalanine concentrations, because PAH requires BH4 as a coenzyme. BH4 is also required for tyrosine hydroxylase and tryptophan hydroxylase, which catalyze reactions leading to the synthesis of neurotransmitters, such as serotonin and the catecholamines. Simply restricting dietary phenylalanine does not reverse the central nervous system effects due to deficiencies in neurotransmitters. Supplementation with BH4 and replacement therapy with L-3,4-dihydroxyphenylalanine and 5-hydroxytryptophan (products of the affected tyrosine hydroxylase– and tryptophan hydroxylase–catalyzed reactions) improves the clinical outcome in these variant forms of hyperphenylalaninemia, although the response is unpredictable.
Figure 20.16 Biosynthetic reactions involving amino acids and tetrahydrobiopterin. [Note: Aromatic amino acid hydroxylases use BH4 and not PLP (pyridoxal phosphate).] NAD(H) = nicotinamide adenine dinucleotide; GTP = guanosine triphosphate; DOPA = 3,4-dihydroxyphenylalanine.
1. Characteristics of classic phenylketonuria:
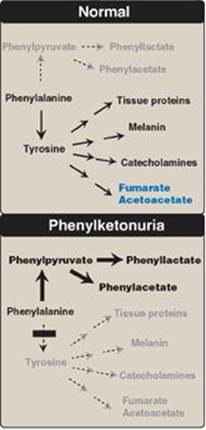
a. Elevated phenylalanine: Phenylalanine is present in high concentrations (ten times normal) in tissues, plasma, and urine. Phenyllactate, phenylacetate, and phenylpyruvate, which are not normally produced in significant amounts in the presence of functional PAH, are also elevated in PKU (Figure 20.17). These metabolites give urine a characteristic musty (“mousey”) odor. [Note: The disease acquired its name from the presence of a phenylpyruvate, a phenylketone in the urine.]
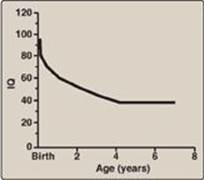
b. Central nervous system symptoms: Severe intellectual disability, developmental delay, microcephaly, and seizures are characteristic findings in untreated PKU. The patient with untreated PKU typically shows symptoms of intellectual disability by age 1 year and rarely achieves an intelligence quotient (IQ) greater than 50 (Figure 20.18). [Note: These clinical manifestations are now rarely seen as a result of neonatal screening programs.]
c. Hypopigmentation: Patients with untreated PKU may show a deficiency of pigmentation (fair hair, light skin color, and blue eyes). The hydroxylation of tyrosine by copper-requiring tyrosinase, which is the first step in the formation of the pigment melanin, is inhibited in PKU.
Figure 20.17 Pathways of phenylalanine metabolism in normal individuals and in patients with phenylketonuria.
2. Neonatal screening and diagnosis: Early diagnosis of PKU is important because the disease is treatable by dietary means. Due to the lack of neonatal symptoms, laboratory testing for elevated blood levels of phenylalanine is mandatory for detection. However, the infant with PKU frequently has normal blood levels of phenylalanine at birth because the mother clears increased blood phenylalanine in her affected fetus through the placenta. Normal levels of phenylalanine may persist until the newborn is exposed to 24–48 hours of protein feeding. Thus, screening tests are typically done after this time to avoid false negatives. For newborns with a positive screening test, diagnosis is confirmed through quantitative determination of phenylalanine levels.
Figure 20.18 Typical intellectual ability in untreated patients of different ages with phenylketonuria. IQ = intelligence quotient.
3. Prenatal diagnosis: Classic PKU is caused by any of 100 or more different mutations in the gene that codes for PAH. The frequency of any given mutation varies among populations, and the disease is often doubly heterozygous (that is, the PAH gene has a different mutation in each allele). Despite this complexity, prenatal diagnosis is possible (see p. 477).
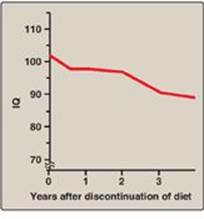
4. Treatment: Most natural protein contains phenylalanine, an essential amino acid, and it is impossible to satisfy the body’s protein requirement without exceeding the phenylalanine limit when ingesting a normal diet. Therefore, in PKU, blood phenylalanine level is maintained close to the normal range by feeding synthetic amino acid preparations free of phenylalanine, supplemented with some natural foods (such as fruits, vegetables, and certain cereals) selected for their low phenylalanine content. The amount is adjusted according to the tolerance of the individual as measured by blood phenylalanine levels. The earlier treatment is started, the more completely neurologic damage can be prevented. Individuals who are appropriately treated can have normal intelligence. [Note: Treatment must begin during the first 7–10 days of life to prevent cognitive impairment.] Because phenylalanine is an essential amino acid, overzealous treatment that results in blood phenylalanine levels below normal is avoided. In patients with PKU, tyrosine cannot be synthesized from phenylalanine, and, therefore, it becomes an essential amino acid and must be supplied in the diet. Discontinuance of the phenyalanine-restricted diet in early childhood is associated with poor performance on IQ tests. Adult PKU patients show deterioration of IQ scores after discontinuation of the diet (Figure 20.19). Lifelong restriction of dietary phenylalanine is, therefore, recommended. [Note: Individuals with PKU are advised to avoid aspartame, an artificial sweetener that contains phenylalanine.]
Figure 20.19 Changes in IQ scores after discontinuation of low-phenylalanine diet in patients with phenylketonuria. IQ = intelligence quotient.
5. Maternal phenylketonuria: If women with PKU who are not on a low-phenylalanine diet become pregnant, the offspring are affected with “maternal PKU syndrome.” High blood phenylalanine levels in the mother cause microcephaly and congenital heart abnormalities in the fetus (phenylalanine is a teratogen). Because these developmental responses to high phenylalanine occur during the first months of pregnancy, dietary control of blood phenylalanine must begin prior to conception and must be maintained throughout the pregnancy.
B. Maple syrup urine disease
Maple syrup urine disease (MSUD) is a rare (1:185,000), autosomal recessive disorder in which there is a partial or complete deficiency in BCKD, a mitochondrial enzyme complex that oxidatively decarboxylates leucine, isoleucine, and valine (see Figure 20.10). These BCAAs and their corresponding α-keto acids accumulate in the blood, causing a toxic effect that interferes with brain functions. The disease is characterized by feeding problems, vomiting, ketoacidosis, changes in muscle tone, neurologic problems that can result in coma (primarily due to the rise in leucine), and a characteristic maple syrup-like odor of the urine due to the rise in isoleucine. If untreated, the disease is fatal. If treatment is delayed, intellectual disability results.
1. Classification: The term “maple syrup urine disease” includes a classic type and several variant forms of the disorder. The classic, neonatal-onset form is the most common type of MSUD. Leukocytes or cultured skin fibroblasts from these patients show little or no BCKD activity. Infants with classic MSUD show symptoms within the first several days of life. If not diagnosed and treated, classic MSUD is lethal in the first weeks of life. Patients with intermediate forms have a higher level of enzyme activity (up to 30% of normal). The symptoms are milder and show an onset from infancy to adolescence. Patients with the rare thiamine-dependent variant of MSUD respond to large doses of this vitamin.
2. Screening and diagnosis: As with PKU, prenatal diagnosis and neonatal screening are available, and most affected individuals are compound heterozygotes.
3. Treatment: MSUD is treated with a synthetic formula that is free of BCAAs, supplemented with limited amounts of leucine, isoleucine, and valine to allow for normal growth and development without producing toxic levels. [Note: Elevated leucine is the cause of the neurologic damage in MSUD, and its level is carefully monitored.] Early diagnosis and lifelong dietary treatment is essential if the child with MSUD is to develop normally. [Note: BCAAs are an important energy source in times of metabolic need, and individuals with MSUD are at risk of decompensation during periods of increased protein catabolism.]
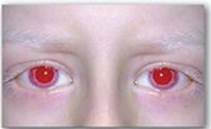
Figure 20.20 Patient with oculocutaneous albinism, showing white eyebrows and lashes and eyes that appear red in color.
C. Albinism
Albinism refers to a group of conditions in which a defect in tyrosine metabolism results in a deficiency in the production of melanin. These defects result in the partial or full absence of pigment from the skin, hair, and eyes. Albinism appears in different forms, and it may be inherited by one of several modes: autosomal recessive (primary mode), autosomal dominant, or X linked. Total absence of pigment from the hair, eyes, and skin (Figure 20.20), tyrosinase-negative oculocutaneous albinism (type 1 albinism), results from an absent or defective copper-requiring tyrosinase. It is the most severe form of the condition. In addition to hypopigmentation, affected individuals have vision defects and photophobia (sunlight hurts their eyes). They are at increased risk for skin cancer.
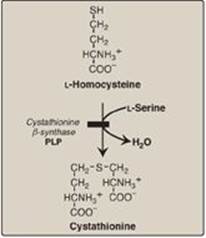
D. Homocystinuria
The homocystinurias are a group of disorders involving defects in the metabolism of Hcy. These autosomal-recessive diseases are characterized by high plasma and urinary levels of Hcy and methionine and low levels of cysteine. The most common cause of homocystinuria is a defect in the enzyme cystathionine β-synthase, which converts Hcy to cystathionine (Figure 20.21). Individuals who are homozygous for cystathionine β-synthasedeficiency exhibit dislocation of the lens (ectopia lentis), skeletal anomalies (long limbs and fingers), intellectual disability, and an increased risk for developing thrombi (blood clots). Thrombosis is the major cause of early death in these individuals. Patients can be responsive or nonresponsive to oral administration of pyridoxine (vitamin B6), which is converted to pyridoxal phosphate, the coenzyme of cystathionine β-synthase. Vitamin B6–responsive patients usually have a milder and later onset of clinical symptoms compared with B6-nonresponsive patients. Treatment includes restriction of methionine intake and supplementation with vitamins B6, B12, and folate.
Figure 20.21 Enzyme deficiency in homocystinuria. PLP = pyridoxal phosphate.
E. Alkaptonuria (alcaptonuria)
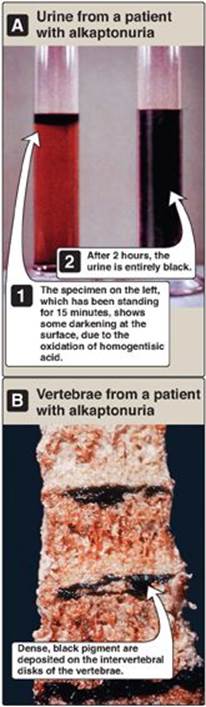
Alkaptonuria is a rare metabolic condition involving a deficiency in homogentisic acid oxidase, resulting in the accumulation of homogentisic acid (HA), an intermediate in the degradative pathway of tyrosine (see p. 269.) The condition has three characteristic symptoms: homogentisic aciduria (the urine contains elevated levels of HA, which is oxidized to a dark pigment on standing, as shown in Figure 20.22A), large joint arthritis, and deposition of black pigment (ochronosis) in cartilage and collagenous tissue (Figure 20.22B). Patients with alkaptonuria are usually asymptomatic until about age 40 years. Dark staining of diapers can indicate the disease in infants, but usually no symptoms are present until later in life. Diets low in phenylalanine and tyrosine reduce the levels of HA and decrease the amount of pigment deposited in body tissues. Although alkaptonuria is not life-threatening, the associated arthritis may be severely crippling.
Figure 20.22 A patient with alkaptonuria. A. Urine. B. Vertebrae.
VII. CHAPTER SUMMARY
Amino acids whose catabolism yields pyruvate or one of the intermediates of the tricarboxylic acid cycle are termed glucogenic (Figure 20.23). They can give rise to the net formation of glucose in the liver and the kidney. The solely glucogenic amino acids are glutamine, glutamate, proline, arginine, histidine, alanine, serine, glycine, cysteine, methionine, valine, threonine, aspartate, and asparagine. Amino acids whose catabolism yields either acetoacetate or one of its precursors, acetyl coenzyme A (CoA) or acetoacetyl CoA, are termed ketogenic. Leucine and lysine are solely ketogenic. Tyrosine, phenylalanine, tryptophan, and isoleucine are both ketogenic and glucogenic. Nonessential amino acids can be synthesized from metabolic intermediates or from the carbon skeletons of essential amino acids. Essential amino acids need to be obtained from the diet. They include histidine, methionine, threonine, valine, isoleucine, phenylalanine, tryptophan, leucine, and lysine. Phenylketonuria (PKU) is caused by a deficiency of phenylalanine hydroxylase (PAH), the enzyme that converts phenylalanine to tyrosine. Hyperphenylalaninemia may also be caused by deficiencies in the enzymes that synthesize or regenerate the coenzyme for PAH, tetrahydrobiopterin. Untreated patients with PKU suffer from severe intellectual disability, developmental delay, microcephaly, seizures and a characteristic mousey smell of the urine. Treatment involves controlling dietary phenylalanine. Tyrosine becomes an essential dietary component for people with PKU. Maple syrup urine disease is caused by a partial or complete deficiency in branched-chain α-keto acid dehydrogenase, the enzyme that decarboxylates leucine, isoleucine, and valine. Symptoms include feeding problems, vomiting, ketoacidosis, changes in muscle tone, and a characteristic sweet smell of the urine. If untreated, the disease leads to neurologic problems that result in death. Treatment involves controlling dietary leucine, isoleucine, and valine. Other important genetic diseases associated with amino acid metabolism include albinism, homocystinuria, methylmalonyl CoA mutase deficiency, alkaptonuria, histidinemia, tyrosinemia, and cystathioninuria.
Figure 20.23 Key concept map for amino acid metabolism. CoA = coenzyme A.
Study Questions:
Choose the ONE best answer.
For Questions 20.1–20.3, match the deficient enzyme with the associated clinical sign or laboratory finding in urine.
A. Black pigmentation of cartilage
B. Cabbage-like odor of fluids
C. Cystine crystals in urine
D. White hair, red eye color
E. Increased branched-chain amino acids
F. Increased homocysteine
G. Increased methionine
H. Increased phenylalanine
20.1 Cystathionine β-synthase
20.2 Homogentisic acid oxidase
20.3 Tyrosinase
Correct answers = F, A, D. A deficiency in cystathionine β-synthase of methionine degradation results in a rise in homocysteine. A deficiency in homogentisic acid oxidase of tyrosine degradation results in a rise in homogentisic acid, which forms a black pigment that is deposited in connective tissue. A deficiency in tyrosinase results in decreased formation of melanin from tyrosine in skin, hair, and eyes. A cabbage-like odor is characteristic of isovaleryl coenzyme A dehydrogenase deficiency. Cystine crystals in urine are seen with cystinuria, a defect in intestinal and renal cystine absorption. Increased branched-chain amino acids are seen in maple syrup urine disease, increased methionine is seen in defects in homocysteine metabolism, and increased phenylalanine is seen in phenylketonuria.
20.4 A 1-week-old infant, who was born at home in a rural area, has undetected classic phenylketonuria. Which statement about this baby and/or her treatment is correct?
A. A diet devoid of phenylalanine should be initiated immediately.
B. Dietary treatment will be recommended to be discontinued in adulthood.
C. Supplementation with vitamin B6 is required.
D. Tyrosine is an essential amino acid.
Correct answer = D. In patients with phenylketonuria, tyrosine cannot be synthesized from phenylalanine and, hence, becomes essential and must be supplied in the diet. Phenylanine in the diet must be controlled but cannot be eliminated entirely because it is an essential amino acid. Dietary treatment must begin during the first 7–10 days of life to prevent intellectual disability, and life-long restriction of phenylalanine is recommended to prevent cognitive decline. Additionally, elevated levels of phenylalanine are teratogenic to a developing fetus.
20.5 Which one of the following statements concerning amino acids is correct?
A. Alanine is ketogenic.
B. Amino acids that are catabolized to acetyl coenzyme A are glucogenic.
C. Branched-chain amino acids are catabolized primarily in liver.
D. Cysteine is essential for individuals consuming a diet severely limited in methionine.
Correct answer = D. Methionine is the precursor of cysteine, which becomes essential if methionine is severely restricted. Alanine is the primary glucogenic amino acid. Acetyl coenzyme A (CoA) cannot be used for the net synthesis of glucose. Amino acids catabolized to acetyl CoA are ketogenic. Branched-chain amino acids are catabolized primarily in skeletal muscle.
20.6 In an individual with the Enzyme 3–deficient form of maple syrup urine disease, why would lactic acidosis be an expected finding?
The three α-keto acid dehydrogenase complexes (pyruvate dehydrogenase [PDH], α-ketoglutarate dehydrogenase, and branched-chain α-keto acid dehydrogenase [BCKD]) have a common Enzyme 3 (E3) (dihydrolipoyl dehydrogenase). In E3-deficient maple syrup urine disease, in addition to the branched-chain amino acids and their α-keto acid derivatives accumulating as a result of decreased activity of BCKD, lactate will also be increased because of decreased activity of PDH.
20.7 In contrast to the vitamin B6-derived pyridoxal phosphate required in most enzymic reactions involving amino acids, what coenzyme is required by the aromatic amino acid hydroxylases?
Tetrahydrobiopterin, made from guanosine triphosphate, is the required coenzyme