Lippincott’s Illustrated Reviews: Biochemistr, Sixth Edition (2014)
UNIT V: Integration of Metabolism
Chapter 26. Obesity
I. OVERVIEW
Obesity is a disorder of body weight regulatory systems characterized by an accumulation of excess body fat. In primitive societies, in which daily life required a high level of physical activity and food was only available intermittently, a genetic tendency favoring storage of excess calories as fat may have had a survival value. Today, however, the sedentary lifestyle and abundance and wide variety of palatable, inexpensive foods in industrialized societies has undoubtedly contributed to an obesity epidemic. As adiposity has increased, so has the risk of developing associated diseases, such as type 2 diabetes (T2D), cardiovascular disease, hypertension, cancer, and arthritis. Particularly alarming is the explosion of obesity in children and adolescents, which has shown a threefold increase in prevalence over the last two decades. [Note: Approximately 17% of those ages 2-19 years are obese.] In the United States, the lifetime risk of becoming overweight or obese is approximately 50% and 25%, respectively. Obesity has increased globally. In fact, by some estimates, there are more obese than undernourished individuals worldwide.
II. ASSESSMENT OF OBESITY
Because the amount of body fat is difficult to measure directly, it is usually determined from an indirect measure, the body mass index (BMI), which has been shown to correlate with the amount of body fat in most individuals. [Note: Exceptions are athletes who have large amounts of lean muscle mass.] Measuring the waist size with a tape measure is also used to screen for obesity, because this measurement reflects the amount of fat in the central abdominal area of the body. The presence of excess central fat is associated with an increased risk for morbidity and mortality, independent of the BMI. [Note: A waist size ≥ 40 inches in men and ≥ 35 inches in women is considered a risk factor.]
A. Body mass index
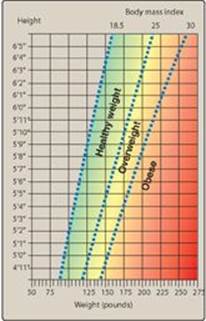
The BMI (weight in kg)/(height in meters)2 provides a measure of relative weight, adjusted for height. This allows comparisons both within and between populations. The healthy range for the BMI is between 18.5 and 24.9. Individuals with a BMI between 25 and 29.9 are considered overweight, those with a BMI equal to or greater than 30 are defined as obese, and a BMI over 40 is considered extremely obese. Anyone more than 100 pounds overweight is considered severely (morbidly) obese (Figure 26.1). These cutoffs are based on the studies examining the relationship of BMI to premature death and are similar in men and women. Nearly two thirds of American adults are overweight, and more than one third of those are obese.
Figure 26.1 Body mass index (BMI) Chart. To use the BMI Chart, find height in the lefthand column. Move across the row to weight. Height and weight intersect at the individual’s BMI. [Note: To calculate BMI using inches and pounds, use BMI = [weight in pounds/ (height in inches)2] x 703.
B. Anatomic differences in fat deposition
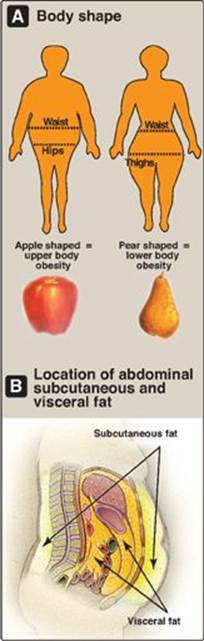
The anatomic distribution of body fat has a major influence on associated health risks. A waist-to-hip ratio of more than 0.8 for women and more than 1.0 for men is defined as android, “apple-shaped,” or upper body obesity, and is associated with more fat deposition in the trunk (Figure 26.2A). In contrast, a lower waist/hip ratio reflects a preponderance of fat distributed in the hips and thighs and is called gynoid, “pear-shaped,” or lower body obesity. It is defined as a waist/hip ratio of less than 0.8 for women and less than 1.0 for men. The pear shape, more commonly found in women, presents a much lower risk of metabolic disease, and some studies indicate that it may actually be protective. Thus, the clinician can use simple indices of body shape to identify those who may be at higher risk for metabolic diseases associated with obesity.
About 80%–90% of the fat stored in the human body is in subcutaneous depots, just under the skin, in the abdominal (upper body) and the gluteal-femoral (lower body) regions. In addition, 10%–20% of body fat is stored in so-called visceral depots (omental and mesenteric), which are located within the abdominal cavity in close association with the digestive tract (Figure 26.2B). Excess fat in visceral and abdominal subcutaneous stores increases health risks associated with obesity.
Figure 26.2 A. Individuals with upper body obesity (left) have greater health risks than individuals with lower body obesity (right). B. Visceral fat is located inside the abdominal cavity, packed in between the internal organs. Subcutaneous fat is found underneath the skin.
C. Biochemical differences in regional fat depots
The regional types of fat described above are biochemically different. Subcutaneous adipocytes from the lower body (gluteal-femoral), particularly in women, are larger, very efficient at fat (triacylglycerol [TAG]) deposition, and tend to mobilize fatty acids more slowly than those from the abdominal subcutaneous depots. Visceral adipocytes are the most metabolically active. Both abdominal subcutaneous and visceral depots of obese subjects have high rates of lipolysis and contribute to increased availability of free fatty acids (FFAs). These metabolic differences may contribute to the higher risk found in individuals with upper body obesity.
1. Endocrine function: White adipose tissue, once thought to be a passive reservoir of TAGs, is now known to play an active role in body weight regulatory systems. For example, the adipocyte is an endocrine cell that secretes a number of protein regulators, such as the hormones leptin and adiponectin. Leptin regulates appetite as well as metabolism (see p. 353). Adiponectin reduces levels of FFAs in the blood and has been associated with improved lipid profiles, increased insulin sensitivity resulting in better glycemic control, and reduced inflammation in diabetic patients. [Note: Adiponectin levels decrease as body weight increases, and leptin levels increase.]
2. Importance of portal circulation: With obesity, there is increased release of FFAs and secretion of proinflammatory cytokines, such as interleukin 6 (IL-6), from adipose tissue. [Note: Cytokines are small proteins that regulate the immune system.] One reason that visceral and abdominal adipose depots may have such a large influence on metabolic dysfunction in obesity is that the FFAs and cytokines released from these depots enter the portal vein and, therefore, have direct access to the liver. In the liver, they may lead to insulin resistance (see p. 342) and increased hepatic synthesis of TAGs, which are released as components of very-low-density lipoprotein particles and contribute to the hypertriacylglycerolemia associated with obesity. By contrast, FFAs from lower body subcutaneous adipose depots enter the general circulation, where they can be oxidized in muscle and, therefore, reach the liver in lower concentration.
D. Size and number of fat cells
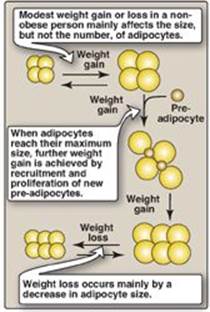
As TAGs are stored, adipocytes can expand to an average of two to three times their normal volume. (Figure 26.3). However, the ability of a fat cell to expand is limited. With prolonged overnutrition, preadipocytes within adipose tissue are stimulated to proliferate and differentiate into mature fat cells, increasing the number of adipocytes. Thus, most obesity is due to a combination of increased fat cell size (hypertrophy) and number (hyperplasia). Like other tissues, the adipose tissue undergoes continuous remodeling. Contrary to early dogma, we now know that adipocytes can die. The estimated average lifespan of an adipocyte is 10 years.
Obese individuals can have up to five times the normal number of fat cells. If excess calories cannot be accommodated within adipose tissue, the excess fatty acids “spill over” into other tissues, such as muscle and liver. The amount of this so-called “ectopic fat” is strongly associated with insulin resistance. With weight loss in an obese individual, the size of the fat cells is reduced, but the number of fat cells is not usually affected. Thus, a normal body fat is achieved by decreasing the size of the fat cell below normal. Small fat cells are very efficient at reaccumulating fat, and this may drive appetite and weight regain.
Figure 26.3 Hypertrophic (increased size) and hyperplastic (increased number) changes to adipocytes are thought to occur in severe obesity.
III. BODY WEIGHT REGULATION
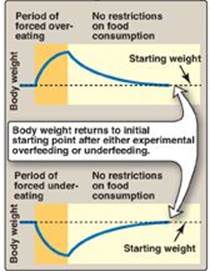
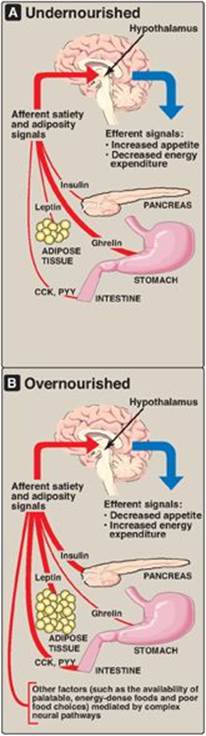
The body weight of most individuals tends to be relatively stable over time. This observation prompted the hypothesis that each individual has a biologically predetermined “set point” for body weight. The body attempts to add to adipose stores when the body weight falls below the set point and to lose adipose from stores when the body weight is higher than the set point. Thus, the body defends the set point. For example, with weight loss, appetite increases and energy expenditure falls, whereas with overfeeding, appetite falls and energy expenditure may slightly increase (Figure 26.4). However, a strict set point model explains neither why some individuals fail to revert to their starting weight after a period of overeating nor the current epidemic of obesity.
A. Genetic contributions to obesity
It is now evident that genetic mechanisms play a major role in determining body weight.
1. Biologic origin: The importance of genetics as a determinant of obesity is indicated by the observation that children who are adopted usually show a body weight that correlates with their biologic rather than adoptive parents. Furthermore, identical twins have very similar BMIs (Figure 26.5), whether reared together or apart, and their BMIs are more similar than those of nonidentical, dizygotic twins.
Figure 26.4 Weight changes following episodes of overfeeding or underfeeding followed by feeding with no restrictions.
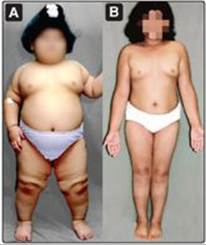
2. Mutations: Rare, single gene mutations can cause human obesity. For example, mutations in the gene for the adipocyte hormone leptin or its receptor produce hyperphagia (increased appetite for and consumption of food) and massive obesity (Figure 26.6), underscoring the importance of the leptin system in regulating human body weight (Section IV). Most obese humans have elevated leptin levels but appear to be resistant to the appetite-regulating effects of this hormone.
Figure 26.5 Identical twins with combined weight of 1,300 pounds. Note similarity in body shape.

B. Environmental and behavioral contributions
The epidemic of obesity occurring over the last decade cannot be simply explained by changes in genetic factors, which are stable on this short time scale. Clearly, environmental factors, such as the ready availability of palatable, energy-dense foods, play a role in the increased prevalence of obesity. Furthermore, sedentary lifestyles encouraged by TV watching, automobiles, computer usage, and energy-sparing devices in the workplace and at home decrease physical activity and enhance the tendency to gain weight. Eating behaviors, such as snacking, portion size, variety of foods consumed, an individual’s unique food preferences, and the number of people present during eating also influence food consumption. It is important to note, however, that in this same environment, many individuals do not become obese. The susceptibility to obesity appears to be explained, at least in part, by an interaction of an individual’s genes and his or her environment and can be influenced by additional factors such as maternal under- or overnutrition that may “set” the body regulatory systems to defend a higher or lower level of body fat. Epigenetic changes (see p. 409), therefore, likely influence the risk for obesity.
IV. MOLECULES THAT INFLUENCE OBESITY
The cause of obesity can be summarized in a deceptively simple statement of the first law of thermodynamics: Obesity results when energy (caloric) intake exceeds energy expenditure. However, the mechanism underlying this imbalance involves a complex interaction of biochemical, neurologic, environmental, and psychologic factors. The basic neural and humoral pathways that regulate appetite, energy expenditure, and body weight involve systems that regulate short-term food intake (meal to meal), and signals for the long-term (day to day, week to week, year to year) regulation of body weight (Figure 26.7).
Figure 26.6 A. Patient with leptin deficiency before initiation of therapy at age 5 years. B. Patient at age 9 years after 48 months of therapy with subcutaneous injection of recombinant leptin.
A. Long-term signals
Long-term signals reflect the status of TAG stores.
1. Leptin: Leptin is an adipocyte peptide hormone that is secreted in proportion to the size of fat stores. When we consume fewer calories than we need, body fat declines, and leptin production from the fat cell decreases. The body adapts by minimizing energy utilization (decreasing activity) and increasing appetite. Unfortunately, in many individuals, the leptin system may be better at preventing weight loss than preventing weight gain. Although a meal or overeating increases leptin and this should, in theory, also dampen appetite (an anorexigenic effect) and prevent overconsumption of calories, other cues that stimulate appetite can apparently overcome the leptin system in many individuals. [Note: Leptin’s effects are mediated through binding to its receptors in the arcuate nucleus of the hypothalamus.]
2. Insulin: Obese individuals are also hyperinsulinemic. Like leptin, insulin acts on hypothalamic neurons to dampen appetite. (See Chapter 23 for the effects of insulin on metabolism.)
B. Short-term signals
Short-term signals from the gastrointestinal tract control hunger and satiety, which affect the size and number of meals over a time course of minutes to hours. In the absence of food intake (between meals), the stomach produces ghrelin, an orexigenic (appetite-stimulating) hormone that drives hunger. As food is consumed, gut hormones, including cholecystokinin (CCK) and peptide YY, among others, induce satiety (an anorexigenic effect), thereby terminating eating, through actions on gastric emptying and neural signals to the hypothalamus. Within the hypothalamus, neuropeptides such as neuropeptide Y (orexigenic) and α-melanocyte–stimulating hormone (α-MSH), which is anorexigenic, and neurotransmitters, such as serotonin and dopamine, are important in regulating hunger and satiety. Long-term and short-term signals interact, insofar as leptin can affect the sensitivity of hypothalamic neurons to short-term signals such as CCK. Thus, there are many and complex regulatory loops that control the size and number of meals in relationship to the status of body fat stores. [Note: α-MSH binds to the melanocortin-4 receptor (MC4R). Loss-of-function mutations to MC4R are associated with early-onset obesity.]
Figure 26.7 Some signals that influence appetite and satiety. CCK = cholecystokinin, PYY = peptide YY.
V. METABOLIC CHANGES IN OBESITY
The primary metabolic effects of obesity include dyslipidemias, glucose intolerance, and insulin resistance expressed primarily in the liver, muscle, and adipose tissue. These metabolic abnormalities reflect molecular signals originating from the increased mass of adipocytes. (see Figure 25.7 and Figure 26.7)
A. Metabolic syndrome
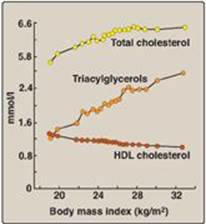
Abdominal obesity is associated with a cluster of metabolic abnormalities that is referred to as the metabolic syndrome and includes glucose intolerance (hyperglycemia below that classified as diabetes; see p. 338), insulin resistance, hyperinsulinemia, dyslipidemia (low levels of high-density lipoprotein and elevated TAGs), and hypertension (Figure 26.8). The metabolic syndrome is also associated with a state of low-grade, chronic, systemic inflammation that contributes to the pathogenesis of insulin resistance and atherosclerosis. In obesity, adipocytes release proinflammatory mediators such as IL-6. Additionally, low levels of the adipocyte hormone adiponectin that normally dampens inflammation and sensitizes tissues, especially the liver, to insulin, may contribute to the metabolic syndrome and, therefore, the risk of T2D and heart disease.
Figure 26.8 Body mass index and changes in blood lipids. HDL= high-density lipoprotein.
VI. OBESITY AND HEALTH
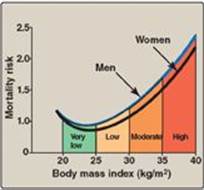
Obesity is correlated with an increased risk of death (Figure 26.9) and is a risk factor for a number of chronic conditions, including T2D, dyslipidemias, hypertension, heart disease, some cancers, gallstones, arthritis, gout, pelvic floor disorders (for example, urinary incontinence), nonalcoholic fatty liver disease, and sleep apnea. The relationship between obesity and associated morbidities is stronger among individuals younger than age 55 years. After age 74 years, there is no longer an association between increased BMI and mortality. Weight loss in obese individuals leads to decreased blood pressure, serum TAGs, and blood glucose levels. High-density lipoproteins increase.
VII. WEIGHT REDUCTION
Weight reduction can help reduce the complications of obesity, including T2D and hypertension. To achieve weight reduction, the obese patient must decrease energy intake or increase energy expenditure, although decreasing energy intake is thought to contribute more to inducing weight loss. Typically, a prescription for weight reduction combines dietary change; increased physical activity; and behavioral modification, which can include nutritional education and meal planning, recording and monitoring food intake through food diaries, modifying factors that lead to overeating, and relearning cues to satiety. Once weight loss is achieved, weight maintenance is a separate process that requires vigilance because the majority of patients regain weight after they stop their weight loss efforts.
A. Physical activity
An increase in physical activity can create an energy deficit. Although adding exercise to a hypocaloric regimen may not produce a greater weight loss initially, exercise is a key component of programs directed at maintaining weight loss. In addition, physical activity increases cardiopulmonary fitness and reduces the risk of cardiovascular disease, independent of weight loss. Persons who combine caloric restriction and exercise with behavioral treatment may expect to lose about 5%–10% of initial body weight over a period of 4–6 months. Studies show that individuals who maintain their exercise program regain less weight after their initial weight loss.
Figure 26.9 Body mass index and the relative risk of death.
B. Caloric restriction
Dieting is the most commonly practiced approach to weight control. Because 1 pound of adipose tissue corresponds to approximately 3,500 kcal, one can estimate the effect that caloric restriction will have on the amount of adipose tissue. Weight loss on calorie-restricted diets is determined primarily by energy intake and not nutrient composition. [Note: Compositional aspects can, however, affect glycemic control and the blood lipid profile.] Caloric restriction is ineffective over the long term for many individuals. More than 90% of people who attempt to lose weight regain the lost weight when dietary intervention is suspended. Nonetheless, it is important to recognize that, although few individuals will reach their ideal weight with treatment, weight losses of 10% of body weight over a 6-month period often reduce blood pressure and lipid levels and enhance control of T2D. The health benefits of even relatively small weight losses should, therefore, be emphasized to the patient.
C. Pharmacologic treatment
Several weight-loss medications are currently approved by the U.S. Food and Drug Administration for use in adults with a BMI of 30 or higher. Three approved for long-term use are: 1) orlistat (decreases absorption of dietary fat), 2) lorcaserin (promotes satiety), and 3) a combination of phentermine (suppresses appetite) and extended-release topiramate (controls seizures). [Note: Phentermine monotherapy is approved for short-term use only.] Their effects on weight reduction tend to be modest, and weight regain upon termination of drug therapy is common.
D. Surgical treatment
Gastric bypass and restriction surgeries are effective in causing weight loss in severely obese individuals. Through mechanisms that remain poorly understood, these operations greatly improve poor blood sugar control in morbidly obese diabetic individuals.
Figure 26.10 Key concept map for obesity.
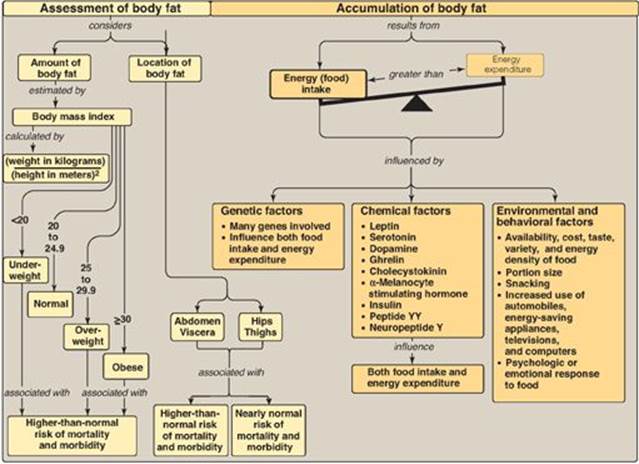
VIII. CHAPTER SUMMARY
Obesity, the accumulation of excess body fat, results when energy (caloric) intake exceeds energy expenditure. Obesity is increasing in industrialized countries because of a reduction in daily energy expenditure and an increase in energy intake resulting from the increasing availability of palatable, inexpensive foods. The body mass index (BMI) is easy to determine and highly correlated to body fat. Nearly two thirds of U.S. adults are overweight(BMI ≥ 25 kg/m2) and more than one third of this group are obese (BMI ≥ 30 kg/m2). The anatomic distribution of body fat has a major influence on associated health risks. Excess fat located in the central abdominal area is associated with greater risk for hypertension, insulin resistance, diabetes, dyslipidemia, and coronary heart disease as compared to fat located in the hips and thighs. A person’s weight is determined by genetic and environmental factors. Appetite is influenced by afferent, or incoming, signals (that is, neural signals, circulating hormones, and metabolites) that are integrated by the hypothalamus. These diverse signals prompt release of hypothalamic peptides and activate outgoing, efferent neural signals. Obesity is correlated with an increased risk of death and is also a risk factor for a number of chronic conditions. Weight reduction is achieved best with negative energy balance, that is, by decreasing caloric intake. Virtually all diets that limit particular groups of foods or macronutrients lead to short-term weight loss. Long-term maintenance of weight loss is difficult to achieve. Modest reduction in food intake occurs with pharmacologic treatment. Surgical procedures, such as gastric bypass, designed to limit food intake are an option for the severely obese patient who has not responded to other treatments.
Study Questions
Choose the ONE best answer.
For Questions 26.1 and 26.2:
A 40-year-old woman, 5 feet, 1 inch (155 cm) tall and weighing 188 pounds (85.5 kg), seeks your advice on how to lose weight. Her waist measured 41 inches and her hips 39 inches. The remainder of the physical examination and the blood laboratory data were all within the normal range. Her only child (who is 14 years old), her sister, and both of her parents are overweight. The patient recalls being overweight throughout her childhood and adolescence. Over the past 15 years, she had been on seven different diets for periods of 2 weeks to 3 months, losing from 5–25 pounds each time. On discontinuation of the diets, she regained weight, returning to 185–190 pounds.
26.1 Calculate and interpret the body mass index for the patient.
Body mass index (BMI) = weight (kg)/height (m2) = 85.5/(1.55)2 = 35.6 kg/m2. Because her BMI is greater than 30, the patient is classified as obese.
26.2 Which one of the following statements best describes the patient?
A. She has approximately the same number of adipocytes as an individual of normal weight, but each adipocyte is larger.
B. She shows an “apple” pattern of fat distribution.
C. She would be expected to show higher-than-normal levels of adiponectin.
D. She would be expected to show lower-than-normal levels of circulating leptin.
E. She would be expected to show lower-than-normal levels of circulating triacylglycerols.
Correct answer = B. Her waist-to-hip ratio is 41/39 = 1.05. Apple shape is defined as a waist/hip ratio of more than 0.8 for women and more than 1.0 for men. She has, therefore, an apple pattern of fat distribution, more commonly seen in males. Compared with other women of the same body weight who have a gynoid (pear-shaped) fat pattern, her android fat pattern places her at greater risk for diabetes, hypertension, dyslipidemia, and coronary heart disease. Individuals with marked obesity and a history dating to early childhood have an adipose depot made up of too many adipocytes, each fully loaded with triacylglycerols (TAGs). Plasma leptin levels are proportional to fat mass, suggesting that resistance to leptin, rather than its deficiency, occurs in human obesity. Adiponectin levels decrease. The elevated circulating fatty acids characteristic of obesity are carried to the liver and converted to TAGs. The TAGs are released as components of very-low-density lipoproteins, resulting in elevated serum TAG levels.