Case Files Biochemistry, 3rd Edition (2015)
SECTION II. Clinical Cases
CASE 7
A 39-year-old woman presents to the clinic for a routine health maintenance examination. The patient reports she is feeling nervous and anxious all the time with frequent palpitations. Upon further questioning, she reports having diarrhea and has been losing weight. She has also noticed a change in hair and fingernail growth and frequently feels warm while others are cold or comfortable. She denies any history of depression or anxiety disorder and is not taking any medications. On examination, her heart rate is 110 beats/minute. She has a slight tremor and has increased reflexes in all extremities. A nontender thyroid enlargement is appreciated in the thyroid region. Her thyroid-stimulating hormone (TSH) level is low at 0.1 mIU/mL. The patient is told that she has an autoimmune antibody process.
![]() What is the most likely diagnosis?
What is the most likely diagnosis?
![]() What is the biochemical mechanism for this disorder?
What is the biochemical mechanism for this disorder?
ANSWERS TO CASE 7:
Hyperthyroidism/Steroid Messenger Regulation of Translation
Summary: A 39-year-old woman has symptoms of nervousness, weight loss, gastrointestinal and skin alterations, heart palpitations, heat intolerance, and physical signs of hyperreflexia and a goiter.
• Likely diagnosis: Hyperthyroidism, likely Graves disease.
• Biochemical mechanism: The most frequent cause of hyperthyroidism, Graves disease, is an autoimmune process in which thyroid hypersecretion is caused by circulating immunoglobulins that bind to the TSH receptor on the thyroid follicular cells and stimulate thyroid hormone production. The diagnosis is confirmed by increased thyroid stimulating immunoglobulin (Ig) G antibodies and is frequently seen in other family members.
CLINICAL CORRELATION
This 39-year-old woman has symptoms of hyperthyroidism, which is an excess of thyroid. This causes a tachycardia, tremor, nervousness, thin skin, weight loss through the hypermetabolic state, and hyperreflexia. If it is unchecked, then the high levels of thyroid hormone can sometimes even cause adrenergic crisis (so-called thyroid storm), which has a high rate of mortality. Normally, the thyroid hormone (thyroxine) is under tight control. The pituitary release of THS is stimulated by insufficient thyroxine, and suppressed by excess thyroxine. In cases of Graves disease, the most common cause of hyperthyroidism in the United States, an autoimmune immunoglobulin is produced that stimulates the TSH receptor of the pituitary. This is confirmed by either assaying for the thyroid-stimulating immunoglobulin, or a radionuclide scan revealing diffuse increased uptake throughout the thyroid gland. Treatment acutely includes β-adrenergic antagonists and agents that inhibit the catabolism of thyroid hormone, such as propylthiouracil (PTU).
APPROACH TO:
Steroid Messenger Regulation of Translation
OBJECTIVES
1. Understand the general mechanisms of hormone action.
2. Know about some of the specific mechanisms by which hormones activate receptors.
a. Know about hormones that bind to cell membrane receptors.
b. Know about hormones that act through cyclic nucleotides.
c. Know about hormones that act through calcium and the PIP2 system.
d. Know about hormones that bind intracellular receptors and activate genes.
3. Be familiar with how immunoglobulins of Graves disease cause hyperthyroidism.
4. Be aware of the mechanism of action of PTU and methimazole.
5. Understand the regulation of hormone levels.
DEFINITIONS
EFFECTOR: The protein in a signal transduction pathway (eg, a hormonal response) that produces the cellular response.
G PROTEIN: A guanosine triphosphate (GTP)–binding protein that serves as a transducer in a signal transduction pathway. Upon binding GTP and releasing guanosine diphosphate (GDP), a G protein is able to activate the effector enzyme (eg, adenylate cyclase).
GRAVES DISEASE: An autoimmune disease in which the B lymphocytes synthesize an immunoglobulin (thyroid-stimulating immunoglobulin [TSIg]) that binds to and activates the TSH receptor in such a way that the thyroid hormones do not feedback inhibit the receptor–effector interaction, leading to a hyperthyroid condition.
HORMONE: A chemical signal that is produced in 1 set of cells and directs the activity in another set of cells that can be endocrine, paracrine, or autocrine.
RECEPTOR: A protein that will perceive the signal of a hormone or other chemical signal (eg, a neurotransmitter) by binding it and transmitting that signal further down the signal transduction pathway.
SECOND MESSENGER: A molecule that is synthesized within a cell in response to a receptor binding a hormone.
TRANSDUCER PROTEIN: A protein (such as the α-subunit of a G protein) that transmits the signal from a hormone-bound receptor to the effector protein.
DISCUSSION
Cellular biochemistry is a complex system of reactions and processes that must be efficiently regulated and integrated with processes underway in other cells and tissues. One method by which regulation is achieved is through the interaction of hormones and their associated receptors located either within or on the surface of the cell. A hormone is any substance in an organism that carries a signal to change metabolic processes within a cell.
The hormonal-signaling process is summarized in Figure 7-1. Hormones are released from secretory tissues in response to metabolic signals as well as electrical or chemical signals from the nervous system. The released hormone binds to a receptor, which can either be on the cell surface or, as in the case of steroid and similar hormones, within the cell. The hormone–receptor complex starts a series of events in which the signal is converted to other chemical forms that bring about changes in the biochemical reactions within the cell.
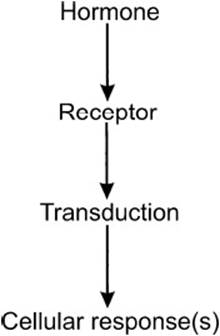
Figure 7-1. The hormonal-signaling process.
Hormones can be grouped into 3 main classifications based on their chemical structure and how they are synthesized. Peptide hormones include the polypeptide hormones insulin and glucagon, as well as the smaller peptides such as thyrotropin-releasing hormone (TRH) and the enkephalins. Some peptide hormones, like thyrotropin (TSH), are glycoproteins. Amino acid-derived hormones are synthesized from amino acid precursors. The catecholamines and serotonin are included in this grouping, as are the thyroid hormones, T3 and T4. The third classification is the steroid and steroid-like hormones, which includes the progestogens, corticosteroids, androgens, estrogens, and calcitriol (the active form of vitamin D).
The cell-associated receptors are the molecular entities primarily responsible for recognizing the hormonal signal. When the hormone binds to the receptor, the hormone–receptor complex initiates the events that culminate in the cellular response. These cell-associated hormone receptors can be classified into 2 main categories, nuclear receptors and cell-surface receptors. Nuclear receptors are intracellular proteins present in either the cytosol or the nucleus that bind hormones, which cross the cell membrane by simple diffusion. When nuclear receptors bind their cognate hormone, they undergo a conformational change that enables them to bind to DNA at specific sitescalled cis-response elements. When the hormone-nuclear receptor binds specifically to these response elements, they influence transcription of the DNA genome into messenger RNA, either by activating or repressing it.
Cell-surface receptors, as their name implies, bind hormones on the extracellular side of the cell membrane. When the hormone binds to its receptor on the cell surface, the hormone-bound receptor either activates or forms a complex with a transducer protein in the membrane that will cause the activation of some enzymatic activity in the cytoplasm of the cell. The enzymatic activity, sometimes called the effector, catalyzes the production of a second messenger that mediates the intracellular response.
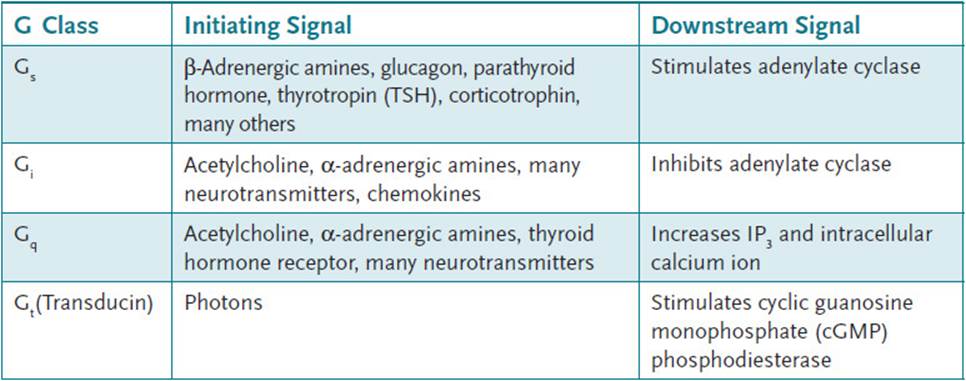
Most transducer proteins that interact with cell-surface receptors are GTP-binding proteins, commonly called G proteins. G proteins typically are composed of 3 subunits: αβ, and γ. When the receptor is in the unbound state, all 3 subunits are bound together to form a heterotrimer that is in close association with the membrane-bound receptor. In this state, GDP is bound to the α-subunit (Figure 7-2). Binding of the hormone by the receptor causes a conformational change such that the α-subunit is able to exchange the bound GDP for a molecule of GTP. The GTP-bound α-subunit separates from the βγ-dimer and can interact with the effector enzyme in either a stimulatory or inhibitory manner, depending on the nature of the α-subunit. Some different G protein families are presented in Table 7-1. Common effector enzymes are adenylate cyclase, which converts ATP into 3′, 5′-cyclic AMP (cAMP) and phospholipase C (PLC), which hydrolyzes the membrane lipid phosphatidylinositol-4,5-bisphosphate (PIP2) to diacylglycerol and inositol-1,4,5-triphosphate (IP3). While GTP is bound to the α-subunit, the interaction with the effector enzyme continues. However, the α-subunit also contains an intrinsic GTPase activity that will hydrolyze GTP to GDP and inorganic phosphate (Pi). This provides a mechanism by which the hormonal signal can be switched off, because GTP hydrolysis to GDP causes the α-subunit to release the effector enzyme and reassociate with the βγ subunits.
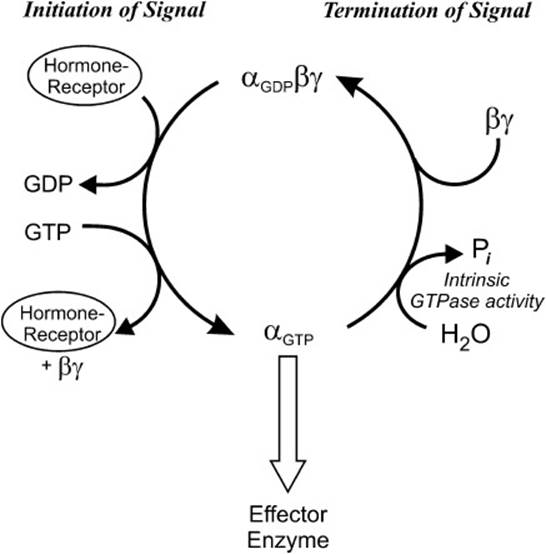
Figure 7-2. Transduction by heterotrimeric G proteins.
Table 7-1 • G-PROTEIN FAMILIES AND THEIR FUNCTIONS
The hormonal cascade known as the hypothalamic-pituitary-thyroid axis employs G-protein transducers that activate both adenylate cyclase and phospholipase C. After receiving an electrochemical signal from the central nervous system (CNS), thyrotropin-releasing hormone (TRH) is released from the arcuate nucleus and median eminence of the hypothalamus. TRH is carried to the anterior pituitary via long portal vessels. In the anterior pituitary, TRH binds to G protein–coupled TRH receptors on the cell surface of thyrotrophs. This activates phospholipase C, releasing both DAG and IP3. DAG activates protein kinase C (PKC), which phosphorylates target proteins. The release of IP3 opens Ca2+ channels in the endoplasmic reticulum, thus releasing Ca2+ from storage and increasing cytoplasmic [Ca2+]. Both of these events result in the increase in synthesis and release of thyrotropin, also known as TSH, which is stored in secretory granules within the thyrotroph. Circulating TSH binds to TSH receptors on the basolateral membranes of the thyroid follicular cell. The TSH receptor is a G protein–coupled receptor that activates adenylate cyclase to produce cAMP when hormone is bound. The increase in cAMP triggers a series of events within the follicular cell that result in the secretion of the thyroid hormones thyroxine (T4; 3,5,3′,5′-tetraiodo-L-thyronine) and 3,5, 3′α-triiodo -L-thyronine (T3). These events include increases in:
1. The transport of iodide ions across the basolateral membrane into the thyroid follicular cell via the Na/I cotransporter (NIS)
2. Iodination of tyrosine residues on colloidal thyroglobulin in the lumen of the follicle
3. Conjugation of iodinated tyrosines to form T3 and T4 on thyroglobulin
4. The endocytosis of thyroglobulin into the follicular cell from the lumen
5. The hydrolysis of thyroglobulin in the lysosome to release T3 and T4
6. The secretion of T3 and T4 into the bloodstream
Although the thyroid hormones are derived from amino acids, they act in a fashion similar to that of the steroid hormones. In the circulation, almost all of the thyroid hormones (99.98% of T4 and 99.5% of T3) are tightly bound to the proteins thyroxine-binding globulin (TBG), albumin, and transthyretin. However, the free, or “unbound,” T3 and T4 are responsible for the biologic effects of the thyroid hormones. The thyroid hormones enter the cell either by simple or carrier-mediated diffusion across the cell membrane. Within the cytoplasm, approximately 50% of T4 is deiodinated to form T3. The hormones enter the nucleus where they bind to the thyroid hormone receptor (THR). The affinity of the THR is approximately 10 times greater for T3 than it is for T4, which is one reason why T3 is more biologically active than T4. When T3 or T4 bind to THR, the complex can bind to the thyroid response element of specific genes. The net effect of increased thyroid hormones is an increase in basal metabolic rate. This is accomplished by increasing the expression of genes that code for enzymes in both the catabolic and anabolic pathways of fats, carbohydrates, and proteins, as well as the expression of the Na-K pump. The careful regulation of thyroid hormone secretion is therefore important in the overall regulation of metabolism. Thus, T3 and T4 exert a negative feedback inhibition on the synthesis of TRH by the hypothalamus and the secretion of TSH by the anterior pituitary, so that the levels of T3 and T4 are tightly controlled.
Graves disease is an autoimmune disease in which the B lymphocytes synthesize Ig that binds to and activates the TSH receptor on the thyroid follicular cell membrane. This TSIg binds to and activates the TSH receptor in such a way that T3 and T4 do not feedback inhibit the receptor–effector interaction. Thus, all of the processes enumerated above that are sequelae of the TSH receptor–binding hormone are increased, resulting in a hyperthyroid condition.
Nonsurgical treatments for Graves disease include administration of thionamide antithyroid drugs, which include propylthiouracil (PTU) and methimazole, and/or treatment with radioactive iodine. The thionamides act by inhibiting the enzyme thyroid peroxidase, an enzyme on the apical membrane of the thyroid follicular cell that faces the lumen. This enzyme catalyzes the oxidation of iodide ion (I–) to atomic iodine (I°), which is required for the iodination of tyrosyl residues on thyroglobulin; these drugs decrease the amounts of T3 and T4 synthesized. They also decrease the amount of TSIg that is synthesized by mechanisms that are not understood. Radioactive iodine treatments cause the progressive destruction of thyroid cells, thus reducing thyroid function over a period of several weeks to months.
COMPREHENSION QUESTIONS
7.1 The thyroid hormones T3 and T4 are synthesized in the follicular cells of the thyroid gland. From which of the following essential amino acids are the thyroid hormones synthesized?
A. Isoleucine
B. Lysine
C. Methionine
D. Phenylalanine
E. Valine
7.2 The thyroid hormones T3 and T4 bind to the THR in the target cells. Which of the following mechanisms best describes the role of the THR?
A. It activates adenylyl cyclase to produce cAMP.
B. It activates the phosphoinositide cascade.
C. It is a soluble guanylyl cyclase.
D. It is a tyrosine kinase.
E. It is a transcription factor.
7.3 A 26-year-old man presents complaining of heat intolerance, heavy sweating, tremulousness, and feeling “jittery inside.” Physical examination reveals reddened conjunctiva and warm and moist palms, but the thyroid gland was not visibly enlarged. Which of the following tests would be most helpful to obtain an accurate diagnosis?
A. Electrocardiography
B. Free thyroxine level
C. Serum cortisol levels
D. Serum electrolytes
E. Serum glucose level
ANSWERS
7.1 D. Thyroid hormones are synthesized from tyrosyl residues on thyroglobulin in the colloidal space of the thyroid follicular cells. Although tyrosine can be obtained from the diet, it can also be synthesized from the essential amino acid phenylalanine by the action of phenylalanine hydroxylase.
7.2 E. Thyroid hormones are similar to steroid hormones in that they bind to receptors in the nucleus of the cell. On binding to the receptor, the receptor–hormone complex binds to DNA affecting the transcription of messenger RNA.
7.3 B. This patient appears to have a hyperthyroid condition, even though the thyroid does not appear to be enlarged. Thyroid function tests would be most helpful to determine if this is the case. The free thyroxine level is a direct measure of the amount of free T4, the biologically active T4, in the serum. Elevation of the free T4 indicates a hyperthyroid condition.
BIOCHEMISTRY PEARLS
![]() Hormones bind to receptors, which can either be on the cell surface or, as in the case of steroid and similar hormones, within the cell.
Hormones bind to receptors, which can either be on the cell surface or, as in the case of steroid and similar hormones, within the cell.
![]() The hormone–receptor complex starts a series of events in which the signal is converted to other chemical forms that bring about changes in the biochemical reactions within the cell.
The hormone–receptor complex starts a series of events in which the signal is converted to other chemical forms that bring about changes in the biochemical reactions within the cell.
![]() Hormones that bind to cell–surface receptors activate or form a complex with a transducer protein in the membrane that will cause the activation of some enzymatic activity in the cytoplasm of the cell, producing a second messenger.
Hormones that bind to cell–surface receptors activate or form a complex with a transducer protein in the membrane that will cause the activation of some enzymatic activity in the cytoplasm of the cell, producing a second messenger.
![]() Nuclear receptors are intracellular proteins present in either the cytosol or the nucleus that bind hormones (steroids), which cross the cell membrane by simple diffusion. The nuclear receptors undergo conformational changes that enable them to bind to DNA at specific sites.
Nuclear receptors are intracellular proteins present in either the cytosol or the nucleus that bind hormones (steroids), which cross the cell membrane by simple diffusion. The nuclear receptors undergo conformational changes that enable them to bind to DNA at specific sites.
REFERENCES
Barrett EJ. The thyroid gland. In: Boron WF, Boulpaep EL, eds. Medical Physiology: A Cellular and Molecular Approach. 2nd ed. Philadelphia, PA: W.B. Saunders; 2011.
Farfel Z, Bourne HR, Iiri T. Mechanisms of disease: the expanding spectrum of G protein diseases. N Engl J Med. 1999;340(13):1012-1020.
Litwack G, Schmidt TJ. Biochemistry of hormones I: polypeptide hormones. In: Devlin TM, ed. Textbook of Biochemistry with Clinical Correlations. 7th ed. New York: Wiley-Liss; 2010.