Case Files Biochemistry, 3rd Edition (2015)
SECTION II. Clinical Cases
CASE 17
A 42-year-old man presents to the emergency department (ED) with complaints of severe epigastric pain and intractable nausea and vomiting for the past 3 days. He reports drinking heavily for the past week but quit drinking and eating a couple of days before his N/V begun. He is currently homeless and is known to be an alcoholic with frequent visits to the ED. He denies any other medical conditions. On examination, he is malnourished, lethargic, and obtunded. He is afebrile but is noted to be slightly hypotensive and tachypneic. His abdominal examination reveals epigastric tenderness but no signs of an acute surgical abdomen. A fruity odor is noticed from his breath. Laboratory values reveal a high anion gap metabolic acidosis with high serum ketone levels. His serum glucose level is noted to be low.
![]() What is the most likely diagnosis?
What is the most likely diagnosis?
![]() What is the initial treatment?
What is the initial treatment?
ANSWERS TO CASE 17:
Alcoholic Ketoacidosis
Summary: A 42-year-old man who abuses alcohols presents to the ED after a binge-drinking episode and a period of fasting with lethargy/epigastric pain, an elevated anion gap metabolic acidosis, and an elevated serum ketone level.
• Diagnosis: Alcoholic ketoacidosis (AKA) results from decreased carbohydrate intake (malnourishment) and alcohol-induced inhibition of gluconeogenesis. The 2 combine to increase free fatty acid delivery to the liver and formation of ketoacids seen in the blood and urine.
• Best treatment: Administration of intravenous (IV) fluids containing dextrose. Thiamine should be given prior to glucose to prevent Wernicke encephalopathy or Korsakoff syndrome. Complications of alcoholic ketoacidosis include seizures, arrhythmias, cardiac arrest, pulmonary edema, and delirium tremens.
CLINICAL CORRELATION
Although ketoacidosis is primarily seen as a complication of uncontrolled diabetes mellitus, there are other causes of this condition. Patients with poor nutrition and alcoholism are also at risk of developing this metabolic derangement. When the body is faced with poor nutrition and decreased carbohydrate intake, the body decreases its insulin secretion and increases its glucagon secretion. The main symptoms are nausea, vomiting, and abdominal pain. Physical examination findings include tachycardia, tachypnea, hypotension, and abdominal distention and tenderness. The arterial blood gas will reveal an anion gap metabolic acidosis, and the glucose level may be normal or decreased. Alcohol inhibits gluconeogenesis and increases lipolysis. The effects from both the malnutrition and alcoholism lead to the formation of ketoacids. Treatment of AKA is based on reversing the 3 key underlying causes: (1) fluid depletion, (2) glycogen depletion, and (3) an elevated ratio of NADH to NAD+. Thus, treatment is with IV fluids containing dextrose increases insulin secretion and decreases glucagon secretion, reversing the underlying biochemical derangement.
APPROACH TO:
Alcoholic Ketoacidosis
OBJECTIVES
1. Describe how ethanol is metabolized by the liver.
2. Explain the mechanism by which consumption of large quantities of alcohol followed by fasting results in a ketoacidotic state.
3. Compare the metabolic profiles of AKA with diabetic ketoacidosis (DKA).
DEFINITIONS
ALCOHOL DEHYDROGENASE: A family of cytosolic isozymes that oxidize alcohols to the corresponding aldehydes using NAD+ as the electron acceptor, converting it to NADH + H+. With ethanol as the substrate, the product is acetaldehyde.
ACETALDEHYDE DEHYDROGENASE: An enzyme that oxidizes acetaldehyde to acetate while converting NAD+ to NADH + H+. The majority of acetaldehyde is metabolized in liver mitochondria (~ 80%) by ALDH2, but there is a cytosolic isozyme (ALDH1) that metabolizes the remainder.
KETOSIS: The metabolic state in which the production of ketone bodies is elevated leading to hyperketonemia, or elevated ketones in the blood.
KETOACIDOSIS: A pathologic metabolic state characterized by uncontrolled ketosis that leads to a lowering of the pH of the blood due to the increased presence of ketoacids. Acetone, a breakdown product of acetoacetate, can usually be detected on the breath.
KETONE BODIES: The metabolites acetoacetate, β-hydroxybutyrate and acetone, which are produced by the catabolism of fatty acids and ketogenic amino acids.
MICROSOMAL ETHANOL OXIDIZING SYSTEM (MEOS): A cytochrome P450 enzyme system located in the endoplasmic reticulum of the liver that oxidizes ethanol to acetaldehyde. The enzyme system consists of cytochrome P450 reductase and CYP2E1, the cytochrome P450 that has the greatest specificity for ethanol. While ethanol is oxidized to acetaldehyde, NADPH is oxidized to NADP+ and O2 is reduced to H2O.
DISCUSSION
Ethanol is a small, amphipathic molecule that is readily absorbed in the intestinal tract by simple passive diffusion and enters the blood stream. The majority of ingested ethanol is metabolized in the liver, although a small percentage (< 10%) is excreted via the breath or urine. As the liver filters ethanol from the bloodstream, hepatocytes oxidize it to acetaldehyde using the cytosolic enzyme alcohol dehydrogenase using NAD+ as the electron accepter to produce NADH as a product (Figure 17-1). Acetaldehyde is a toxic product and is further metabolized to acetic acid. The acetaldehyde must first enter the mitochondrion where an enzyme called acetaldehyde dehydrogenase oxidizes it to acetic acid while reducing NAD+ to NADH (Figure 17-2). Thus, both steps in the conversion of ethanol to acetic acid result in the production of NADH at the expense of NAD+, thereby increasing the [NADH]/[NAD+] ratio in the liver. Although the acetate produced can be activated to acetyl-CoA in the liver and used for energy generation, most of it is secreted into the blood stream, which delivers it to muscle tissue, where it can be activated to acetyl-CoA and completely oxidized to CO2 via the tricarboxylic acid (TCA) cycle and generate ATP via oxidative phosphorylation.
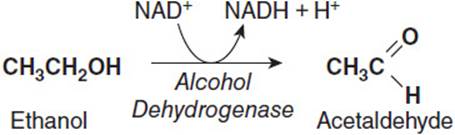
Figure 17-1. Conversion of ethanol to acetaldehyde by alcohol dehydrogenase.
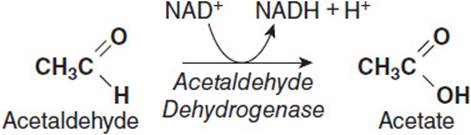
Figure 17-2. Conversion of acetaldehyde to acetate by acetaldehyde dehydrogenase.
The liver also has an alternate system that accounts for 10%-20% of the metabolism of ethanol. The MEOS is located in the endoplasmic reticulum and utilizes a cytochrome P450 enzyme system to convert ethanol to acetaldehyde. The MEOS is a mixed function oxidase enzyme complex that is bound to the endoplasmic reticulum membrane (Figure 17-3). The complex consists of cytochrome P450 reductase and cytochrome P450 2E1 (CYP2E1), and together they oxidize ethanol to acetaldehyde and reduce molecular oxygen to water. The additional reducing equivalents needed for the reaction are obtained by cytochrome P450 reductase converting NADPH to NADP+ and passing those electrons to CYP2E1.
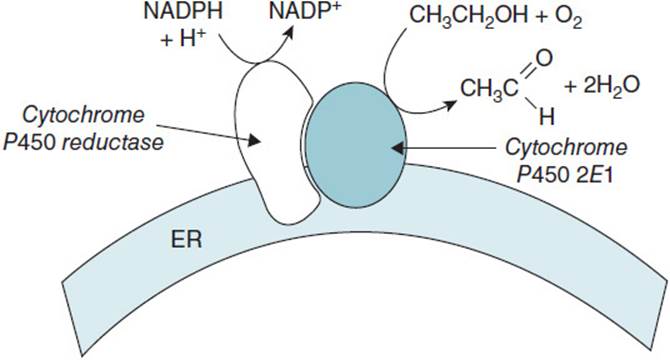
Figure 17-3. The microsomal ethanol oxidizing system.
AKA is a sequela of the increased [NADH]/[NAD+] from metabolism of ethanol and low blood glucose levels from decreased food intake. Most patients with AKA have just had a period of binge drinking. Because of repeated vomiting and epigastric pain, they have refrained from eating, resulting in a low blood glucose concentration and the depletion of stored liver glycogen. The increased [NADH]/[NAD+] from alcohol metabolism impairs gluconeogenesis by inhibiting the conversion of lactate to pyruvate and glycerol to dihydroxyacetone phosphate (DHAP), processes that both require NAD+. Pyruvate and DHAP are both major sources of carbon for the synthesis of glucose via gluconeogenesis.
Low blood glucose from decreased food intake promotes the secretion of glucagon and lipolytic hormones, which stimulate the release of free fatty acids from stored triglyceride in adipose into the bloodstream. After being taken up by the liver, the free fatty acids are activated to fatty acetyl-CoA. Although the rate of β-oxidation is decreased due to the high [NADH]/[NAD+], the acetyl-CoA produced from the fatty acids that do undergo β-oxidation is directed to the production of the ketone bodies acetoacetate and β-hydroxybutyrate. This is because the high [NADH]/[NAD+] in the mitochondrion favors the reduction of oxaloacetate to malate, making it less available for condensation with acetyl-CoA to form citrate. To liberate free CoA for further reactions, 2 molecules of acetyl-CoA recombine to form acetoacetyl-CoA, which is then converted to acetoacetate and β-hydroxybutyrate (Figure 17-4). The excess production of these ketone bodies leads to the ketoacidosis. While in the bloodstream, acetoacetate can undergo a spontaneous, nonenzymatic decarboxylation to yield acetone, which is exhaled and can often be smelled on the breath.
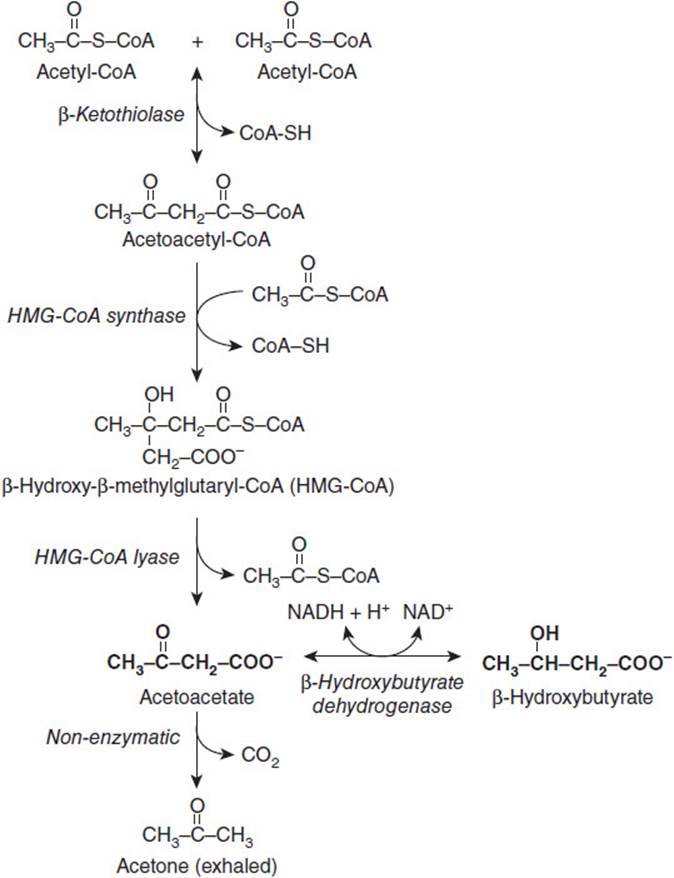
Figure 17-4. Metabolic pathway for the formation of ketone bodies.
While patients that present with DKA or AKA have similarities in their biochemical profiles, there are some important differences. In addition to elevated ketone bodies and decreased blood pH, patients that present usually exhibit a severe depression of insulin levels along with increases in glucagon, cortisol and catecholamines. For patients with DKA, this altered hormonal profile usually results in hyperglycemia due to increased glucose production from gluconeogenesis and decreased uptake of glucose by peripheral tissues. However, with AKA, blood glucose levels are usually low due to decreased nutrient intake because of nausea and vomiting. The increased [NADH]/[NAD+] that results from metabolizing large quantities of ethanol depresses gluconeogenesis. The elevated [NADH]/[NAD+] also leads to a higher lactate/pyruvate ratio and an increased ratio of β-hydroxybutyrate/acetoacetate than is observed with DKA. Because patients with AKA have a higher β-hydroxybutyrate/acetoacetate ratio, the amount of ketone bodies in urine can be underestimated if only tested by ketone test strips, which only measure acetoacetate. Using these ketone test strips to monitor treatment can also give the false impression that ketoacidosis is worsening, when in reality β-hydroxybutyrate is being oxidized back to acetoacetate as the ratio of NADH/NAD+ returns to normal.
COMPREHENSION QUESTIONS
Questions 17.1 to 17.3 refer to the following clinical scenario:
A disoriented man with severe nausea and vomiting and reeking of alcohol is brought to the ED by two persons who found him collapsed on their front lawn. Laboratory samples were taken during his examination and revealed very low blood glucose levels and elevated serum ketone levels.
17.1 Which of the following is the most probable cause of the elevated ketone and low glucose readings?
A. Increased flux of acetyl CoA through the TCA cycle
B. Inadequate dietary intake
C. Increased protein mobilization
D. Increased pyruvate carboxylase activity
17.2 The disorientation observed in the patient is most likely caused by which of the following?
A. Alcohol damage to the kidney
B. Decreased lung surfactant efficacy caused by excessive alcohol intake
C. Increased ketone bodies stimulate diuresis
D. Mentation is suppressed by elevated ketone bodies
E. Neuronal and glial metabolism is reduced
17.3 The patient is treated by IV thiamine and glucose solutions. Although the patient appears to be improving, ketone test strip analysis indicates an increase in urinary ketones. This is best explained by which of the following?
A. Conversion of β-hydroxybutyrate to acetoacetate
B. Decreased activity of the TCA cycle
C. Increased catabolism of ketogenic amino acids
D. Inhibition of pyruvate dehydrogenase
E. Conversion of lactate to pyruvate
17.4 Alcohol metabolism is accomplished in various cellular compartments including the cytosol, mitochondria, and endoplasmic reticulum. Which reaction is least likely to inhibit the synthesis of glucose?
A. Ethanol → Acetaldehyde by cytosolic alcohol dehydrogenase
B. Ethanol → Acetaldehyde by the microsomal ethanol oxidizing system
C. Acetaldehyde → Acetic acid by mitochondrial acetaldehyde dehydrogenase
D. β-Hydroxybutyrate → Acetoacetate
ANSWERS
17.1 B. Increased pyruvate carboxylase would favor increased gluconeogenesis. Increased protein mobilization would also favor an increase in glucose synthesis. Increasing the flux of acetyl CoA through the TCA cycle would decrease the production of ketone bodies. A lack of adequate nutrition due to nausea and vomiting as a result of excessively consuming alcohol would lead to low blood glucose. Gluconeogenesis is slowed due a high NADH/NAD+, but fatty acids continue to be oxidized to elevate ketone body production.
17.2 E. None of the first 4 alternatives affects brain function. Neurons and glia require glucose as the sole source for energy production. With low blood glucose as a result of repressed gluconeogenesis, neurons and glia lack the energy that is required for function.
17.3 A. The increase in ketone bodies detected by ketone test strips is most likely due to conversion of β-hydroxybutyrate to acetoacetate as the NADH/NAD+ returns to normal levels. Treatment with IV glucose solutions will resolve the low concentration of blood glucose, which will decrease the need for β-oxidation of fatty acids and the production acetyl CoA, ketone bodies, and NADH. As the NADH/NAD+ decreases, the equilibrium between acetoacetate and β-hydroxybutyrate shifts toward acetoacetate.
17.4 B. The conversion of ethanol to acetaldehyde by alcohol dehydrogenase, acetaldehyde to acetic acid, and β-hydroxybutyrate to acetoacetate all produce NADH + H+ as products. Increased NADH/NAD+ inhibits those reactions that require NAD+ as an electron acceptor. Several reactions in gluconeogenesis require NAD+; therefore, these reactions would be inhibited by low NAD+. The MEOS does not produce NADH; rather, it requires NADPH as a source of reducing equivalents. Thus, oxidation of ethanol to acetaldehyde by the MEOS would not inhibit gluconeogenesis.
BIOCHEMISTRY PEARLS
![]() Poor nutrition and decreased carbohydrate consumption leads to an increase in glucagon secretion and decreased insulin secretion.
Poor nutrition and decreased carbohydrate consumption leads to an increase in glucagon secretion and decreased insulin secretion.
![]() The increased [NADH]/[NAD+] from alcohol metabolism impairs gluconeogenesis by inhibiting the conversion of lactate to pyruvate and glycerol to DHAP.
The increased [NADH]/[NAD+] from alcohol metabolism impairs gluconeogenesis by inhibiting the conversion of lactate to pyruvate and glycerol to DHAP.
![]() Compared with DKA, glucose levels in AKA are low.
Compared with DKA, glucose levels in AKA are low.
REFERENCES
Umpierrez GE, DiGirolamo M, Tuvlin JA, et al. Differences in metabolic and hormonal milieu in diabetic- and alcohol-induced ketoacidosis. J Crit Care. 2000;15(2):52-59.
Ngatchu T, Sangwaiya A, Dabiri A, et al. Alcoholic ketoacidosis with multiple complications: a case report. Emerg Med J. 2007;24(11):776-777.