Case Files Biochemistry, 3rd Edition (2015)
SECTION II. Clinical Cases
CASE 23
A 2-year-old black girl is being seen by the hematologist after her pediatrician found her to be severely anemic with splenomegaly and jaundice. Her mother gives a possible history of a “blood problem” in her family but does not know for sure. Hemoglobin electrophoresis was normal, and a complete blood count (CBC) revealed normocytic anemia. The platelet and white blood cell counts are normal. On the peripheral smear, there are many bizarre erythrocytes, including spiculated cells. A diagnosis of pyruvate kinase deficiency is made.
![]() What is the biochemical mechanism for this disorder?
What is the biochemical mechanism for this disorder?
![]() How is this disorder inherited?
How is this disorder inherited?
ANSWERS TO CASE 23:
Hemolytic Anemia
Summary: A 2-year-old black girl has normocytic anemia, jaundice, splenomegaly, and peripheral smear showing spiculated cells. A family history of similar symptoms is possible.
• Biochemical mechanism: Pyruvate kinase deficiency usually will manifest clinical symptoms on red blood cells (RBCs) with no apparent metabolic abnormalities in other cells. Insufficient adenosine triphosphate (ATP) is produced in the red cell and its membrane is affected, is rigid, and is removed by the spleen.
• Inheritance: Autosomal recessive.
CLINICAL CORRELATION
Hemolytic anemia is not a common cause of anemia, but should be considered in patients with elevated serum bilirubin or urine bilirubin levels. Lysis of the erythrocyte can occur from various mechanisms such as medications, antibodies against red blood cells, infection, coagulopathy, and mechanical processes such as abnormal heart valves, and enzyme deficiencies of the RBC. Patients may notice fatigue, dizziness from the anemia, and dark colored (classically “cola-colored”) urine from the bilirubinuria.
Confirmation of hemolysis can be obtained by the peripheral blood smear revealing fragmented RBCs, or increased serum bilirubin or decreased serum haptoglobin. Immunoglobulins can cause RBC lysis by attacking various proteins on the surface of erythrocytes, autoimmune processes (body attacking itself), or alloimmune (immunoglobulins from outside) such as from a blood transfusion or a fetus from the mother. The Coombs tests can assess for immunoglobulin on the RBC or circulating in the serum. Typically, hemolysis of the erythrocyte is associated with increased levels of RBC precursors in the bone marrow and, thus, immature forms of the erythrocytes in the bloodstream; therefore, an increased reticulocyte concentration supports the increased destruction of RBCs.
APPROACH TO:
Pyruvate Metabolism
OBJECTIVES
1. Understand the role of pyruvate kinase in pyruvate metabolism.
2. Be familiar with the Embden-Meyerhof pathway of RBC metabolism.
3. Know how pyruvate kinase deficiency results in anemia.
DEFINITIONS
HEMOLYTIC ANEMIA: A pathologic condition in which there is an abnormally lowered number of circulating RBCs caused by rupture of RBCs as a result of membrane abnormalities or deficient enzyme(s) level within the RBC.
GLUCOSE 6-PHOSPHATE DEHYDROGENASE: The enzyme that catalyzes the rate regulating step of the hexose monophosphate shunt, which produces reduced nicotinamide adenine dinucleotide phosphate (NADPH) required for inactivating oxygen radicals and thereby protects the RBC membrane from radical attack and rupture.
PYRUVATE KINASE: Last ATP-producing step in glycolysis and critical in the RBC for maintaining energy supply (ATP levels).
METHEMOGLOBIN REDUCTASE: RBC enzyme that uses NADH to convert the iron of oxidized hemoglobin (methemoglobin) from the ferric (Fe3+) to reduced ferrous state (Fe2+) hemoglobin, which alone is capable of binding oxygen.
DISCUSSION
Hemolytic anemia has many causes, although this case has the marks of undersupply of RBCs caused by an enzyme deficiency within the RBC rather than a membrane abnormality or an environmental factor such as an autoantibody or mechanical trauma.
The RBC in the course of its maturation loses its mitochondria, ribosomes, and nucleus and consequently the functions associated with those organelles such as new enzyme synthesis and mitochondrial energy formation. Thus, the enzyme endowment present at maturation of the RBC cannot be replaced. In terms of energy production in the RBC, only the glycolytic pathway (Embden-Meyerhof pathway) is available. This pathway, together with the hexose monophosphate shunt (pentose phosphate pathway; Figure 23-1), is the only metabolic pathway that uses glucose in RBCs. As a consequence of this limited metabolic capacity, glucose is the only fuel usable by the RBC to generate ATP. Glucose metabolism is required in the RBC to maintain the ionic milieu within the cell, to maintain the heme cofactor of hemoglobin in its reduced (Fe2+) state, to maintain reduced sulfhydryl groups, to maintain the shape of the RBC plasma membrane and to produce 2,3-bisphosphoglycerate, a modulator of hemoglobin affinity for oxygen. To accomplish these tasks approximately 90% of the glucose in the RBC is metabolized directly to pyruvate/lactate while approximately 10% is first metabolized through the hexose monophosphate shunt before reentry into the glycolytic pathway.
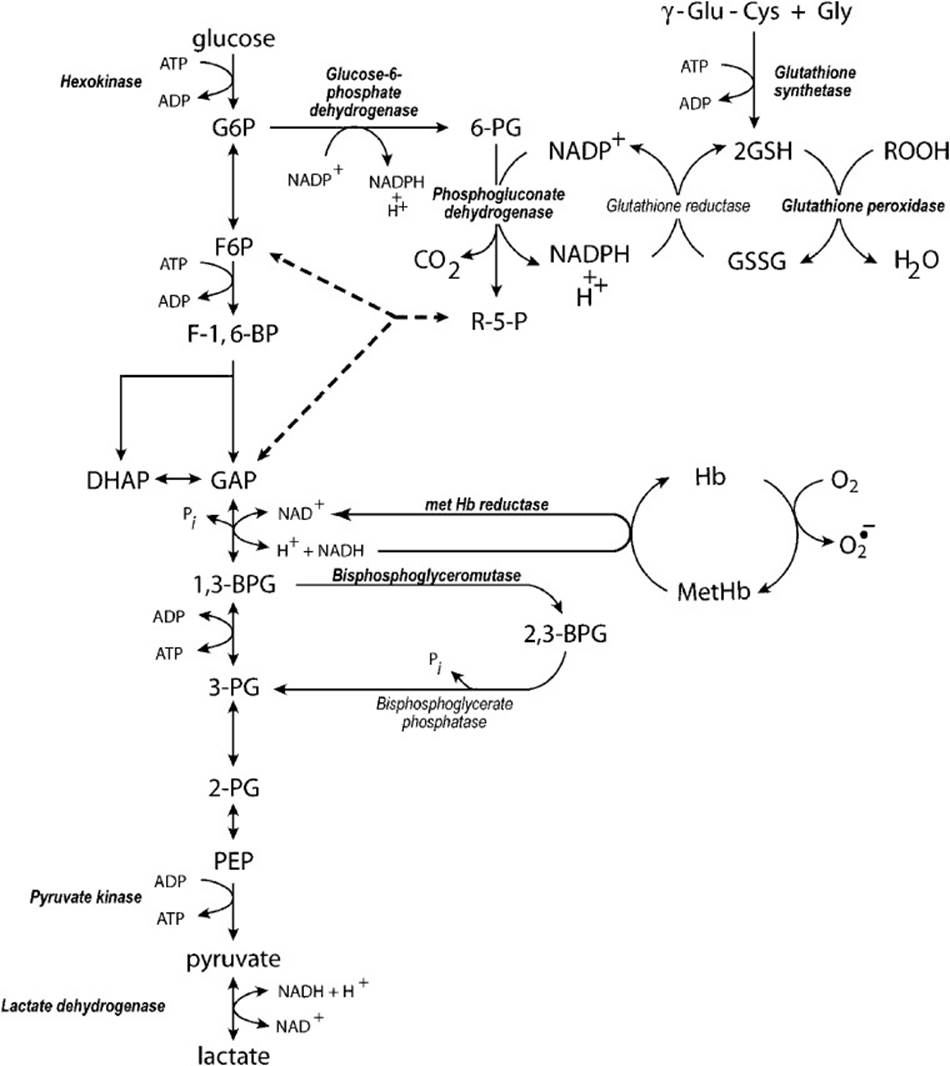
Figure 23-1. Metabolism of glucose in erythrocytes. The following abbreviations are used: G6P = glucose 6-phosphate, 6-PG = 6-phosphogluconate, γ-Glu-Cys = γ-glutamyl-cysteine, Gly = glycine, GSH = reduced glutathione, GSSG = glutathione disulfide, ROOH = organic hydroperoxides, F6P = fructose 6-phosphate, R-5-P = rebulose-5-phosphate, F-1,6-BP = fructose 1, 6-bisphosphate, DHAP = dihydroxyacetone phosphate, GAP = glyceraldehyde 3-phosphate, Hb = hemoglobin (Fe+2), metHb = hemoglobin (Fe+3), 1,3-BPG = 1,3-bisphosphoglycerate, 2,3-BPG, 2,3-bisphosphoglycerate, 3-PG = 3-phosphoglycerate, 2-PG = 2-phosphoglycerate, PEP = phosphoenolpyruvate.
Approximately 90% of all cases of known RBC enzyme deficiencies involve either altered protein or decreased protein levels of pyruvate kinase, whereas 4% are variants of glucose 6-phosphate isomerase, which converts glucose 6-phosphate to fructose 6-phosphate. Most of these enzyme deficiencies are inherited in an autosomal recessive pattern.
As shown in Figure 23-1, 2 mol of ATP are formed per mole of glucose metabolized by the glycolytic cycle. The primary final product of glucose metabolism by glycolysis in the RBC is not pyruvate, as in other tissues most of the time, but lactate. Because RBCs have no mitochondria, NAD+ cannot be regenerated by shuttling NADH produced in glycolysis into the mitochondrial electron transport system. Therefore, the only option to continue glycolysis is to regenerate NAD+ from NADH by reducing pyruvate to lactate in the lactate dehydrogenase reaction. But lactate is not the sole product of RBC glycolysis. Methemoglobin reductase uses some of the NADH produced by glycolysis to reduce methemoglobin (Fe3+) back to active hemoglobin (Fe2+) capable of binding oxygen for transport to the tissues. Thus, the final products are a mixture of lactate and pyruvate with lactate being the primary product.
In tissues other than the RBC, pyruvate has alternative metabolic fates that, depending on the tissue, include gluconeogenesis, conversion to acetyl-CoA by pyruvate dehydrogenase for further metabolism to CO2 in the tricarboxylic acid (TCA) cycle, transamination to alanine or carboxylation to oxaloacetate by pyruvate carboxylase (Table 23-1). However, in the RBC, the restricted enzymatic endowment precludes all but the conversion to lactate. The pyruvate and lactate produced are end products of RBC glycolysis transported out of the RBC to the liver where they can undergo the alternative metabolic conversions described above.
Table 23-1 • METABOLIC FATE OF PYRUVATE
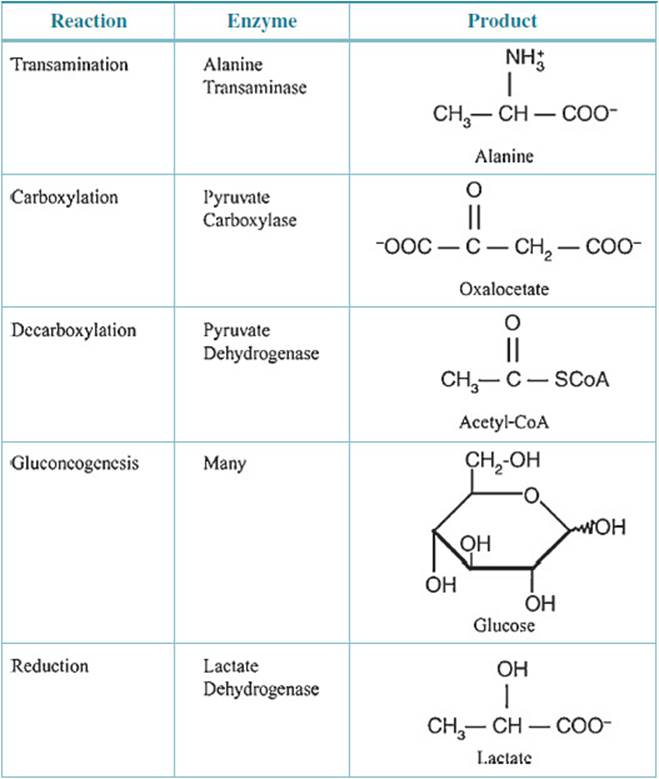
How does compromise of pyruvate kinase activity lead to anemia? Pyruvate kinase lies at the end of the glycolytic pathway in RBCs followed only by lactate dehydrogenase. In any linked pathway where the product of one reaction is the substrate for the next reaction a compromise in one reaction affects the entire pathway. The RBC depends exclusively on glycolysis to produce ATP to discharge all energy-requiring tasks. Pyruvate kinase activity is critical for the pathway and therefore critical for energy production. If ATP is not produced in amounts sufficient to meet the energy demand, then those functions are compromised. Energy is required to maintain the Na+/K+ balance within the RBC and to maintain the flexible discoid shape of the cell. In the absence of sufficient pyruvate kinase activity and therefore ATP, the ionic balance fails, and the membrane becomes misshapen. Cells reflecting pyruvate kinase insufficiency rather than a change in membrane composition are removed from the circulation by the macrophages of the spleen. This results in an increased number of circulating reticulocytes and possibly bone marrow hyperplasia, which is a biologic response to lowered RBC count as a result of hemolysis of erythrocytes.
COMPREHENSION QUESTIONS
23.1 A young man with normocytic anemia, jaundice, and splenomegaly was diagnosed as having RBC pyruvate kinase deficiency after a peripheral blood smear showed spiculated cells. Because pyruvate kinase is abnormal in this patient, not only is less pyruvate made, but intermediates above pyruvate in the glycolytic pathway also build up, slowing the pathway. Which of the following products may not be made in the appropriate amounts in the RBC because of the deficiency of pyruvate?
A. Glucose
B. Oxaloacetate
C. Acetyl-CoA
D. Lactate
23.2 In the RBCs of the patient described above, which of the following would be expected?
A. ADP to ATP ratios would be elevated above normal.
B. NADP+ would increase relative to NADPH.
C. Ribulose 5-phosphate levels would decrease.
D. NADH to NAD+ ratios would decrease.
E. Methemoglobin levels would increase.
23.3 The glycolytic pathway is a multistep process by which glucose is broken down to a 3-carbon metabolite. Some of the steps are listed below:
1. Conversion of 3-phosphoglycerate to 2-phosphoglycerate
2. Conversion of phosphoenolpyruvate to pyruvate
3. Conversion of glyceraldehyde 3-phosphate to 1,3-bisphosphoglycerate
4. Conversion of glucose to glucose 6-phosphate
5. Conversion of fructose 6-phosphate to fructose 1,6-bisphosphate
Which of the following is the correct order of these conversions?
A. 4 → 5 → 1 → 2 → 3
B. 4 → 3 → 1 → 2 → 5
C. 4 → 5 → 3 → 1 → 2
D. 4 → 1 → 3 → 5 → 2
E. 4 → 5 → 3 → 2 → 1
ANSWERS
23.1 D. The RBC has no mitochondria so glucose cannot be made from pyruvate or acetyl-CoA or oxaloacetate. The RBC does have lactate dehydrogenase and conversion to lactate depends on pyruvate levels.
23.2 A. In the RBC a deficiency of pyruvate kinase would tend to shunt glucose toward the hexose monophosphate pathway increasing ribulose 5-P levels, and the ratio of NADP+ to NADPH would decrease. NADH to NAD+ ratios would increase as a result of lower pyruvate levels making more NADH available to reduce methemoglobin and regenerate NAD+. Because pyruvate kinase is deficient, the last ATP formation site is compromised, and so is the formation of ATP in the RBC, elevating the ADP to ATP ratio.
23.3 C. Glucose to glucose 6-phosphate → fructose 6-phosphate → fructose 1,6-bisphosphate → glyceraldehyde 3-phosphate → 1,3-bisphosphoglycerate → 3-phosphoglycerate → 2-phosphoglycerate → phosphoenolphosphate → pyruvate.
BIOCHEMISTRY PEARLS
![]() The enzyme endowment present at maturation of the RBC cannot be replaced, and only the glycolytic pathway (Embden-Meyerhof pathway) is available for energy production in the RBC.
The enzyme endowment present at maturation of the RBC cannot be replaced, and only the glycolytic pathway (Embden-Meyerhof pathway) is available for energy production in the RBC.
![]() Glucose metabolism is required in the RBC to maintain the ionic milieu within the cell, the vast majority via conversion to lactate.
Glucose metabolism is required in the RBC to maintain the ionic milieu within the cell, the vast majority via conversion to lactate.
![]() The vast majority of RBC enzyme deficiencies involves either altered protein or decreased protein levels of pyruvate kinase.
The vast majority of RBC enzyme deficiencies involves either altered protein or decreased protein levels of pyruvate kinase.
![]() Insufficient pyruvate kinase activity compromises erythrocyte ATP production, leading to ionic imbalance and misshaped cell membranes. These cells are removed from the circulation by the macrophages of the spleen.
Insufficient pyruvate kinase activity compromises erythrocyte ATP production, leading to ionic imbalance and misshaped cell membranes. These cells are removed from the circulation by the macrophages of the spleen.
![]() Pyruvate kinase catalyzes 1 of the 3 irreversible steps in the glycolytic pathway, the others being the phosphorylation of glucose to glucose 6-phosphate and the phosphorylation of fructose 6-phosphate to fructose 2,6-bisphosphate.
Pyruvate kinase catalyzes 1 of the 3 irreversible steps in the glycolytic pathway, the others being the phosphorylation of glucose to glucose 6-phosphate and the phosphorylation of fructose 6-phosphate to fructose 2,6-bisphosphate.
REFERENCE
Longo D, Fauci AS, Kaspar D, et al, eds. Harrison’s Principles of Internal Medicine. 18th ed. New York: McGraw-Hill; 2011.