Case Files Biochemistry, 3rd Edition (2015)
SECTION II. Clinical Cases
CASE 26
A 56-year-old man presents to your clinic for follow-up on his diabetes. He has had diabetes since the age of 12 years and has always required insulin for therapy. He reports feeling very tremulous and diaphoretic at 2 AM with the blood sugars in the range of 40 mg/dL, which is very low. However, he notes that his morning fasting blood sugar level is high without taking any carbohydrates. His physician describes the morning high sugars as a result of biochemical processes in response to the nighttime hypoglycemia.
![]() What are the biochemical processes that govern the response to the nighttime hypoglycemia?
What are the biochemical processes that govern the response to the nighttime hypoglycemia?
ANSWER TO CASE 26:
Somogyi Effect
Summary: A 56-year-old man with a long history of insulin diabetes with evening hypoglycemia and fasting morning hyperglycemia.
• Biochemical mechanism of hypoglycemia: The low nighttime serum blood sugar stimulates the counter-regulatory hormones to try to raise the glucose level. These include epinephrine, glucagon, cortisol, and growth hormone, which affect the glucose level and raise it by the time morning comes around.
CLINICAL CORRELATION
This individual has a classic manifestation of the Somogyi effect, which is fasting morning hyperglycemia in response to hypoglycemia in the early morning and late night hours. The danger is that if nighttime blood glucose levels are not measured, the physician may interpret the patient as having hyperglycemia and require even higher doses of insulin. This would be exactly the wrong treatment, because the hypoglycemia is leading to counter-regulatory hormone reaction, and a very low sugar level bound to the high level in the morning. The diagnosis is established by measuring a 2 am glucose level, and when confirmed, then the bedtime neutral protamine Hagedorn (NPH) insulin (intermediate to long-acting) must be decreased.
APPROACH TO:
Glucose and Counter-Regulatory Hormones
OBJECTIVES
1. Describe the regulation of glycogen and glucose production.
2. Explain how insulin and epinephrine affect glucose levels.
3. Summarize the regulation of glucagons.
4. Describe diabetic ketoacidosis and its biochemical mechanism.
DEFINITIONS
EPINEPHRINE: Adrenaline; a catecholamine hormone derived from the amino acids phenylalanine or tyrosine that is synthesized and secreted by the adrenal medulla in response to stress.
GLUT 2: Glucose transporter isoform 2; a transport protein located on the plasma membrane of liver, pancreas, intestine, and kidney that will allow glucose to cross the membrane depending on the concentration gradient. GLUT 2 is the transporter that enables export of glucose from the liver.
KETOACIDOSIS: An elevation of the ketone body concentration that decreases the pH of the arterial blood to a pathologic condition.
KETOGENESIS: The production of ketone bodies by the liver in response to increased β-oxidation with a decreased rate of the Krebs cycle as a result of shuttling C4 acids from the mitochondrion for the synthesis of glucose via gluconeogenesis.
KETONE BODIES: Acetoacetate, β-hydroxybutyrate, and acetone. Acetoacetate and β-hydroxybutyrate are formed by liver enzymes that condense molecules of acetyl-CoA, thus regenerating CoA for continual use in β-oxidation of fatty acids. Acetone is a spontaneous decomposition product of acetoacetate. Ketone bodies are exported from the liver and can be used by some extrahepatic tissues for energy generation.
OXIDATIVE PHOSPHORYLATION: The process by which adenosine triphosphate (ATP) is synthesized from a hydrogen ion gradient across the mitochondrial inner membrane. The hydrogen ion gradient is formed by the action of protein complexes in the mitochondrial membrane that sequentially transfer electrons from the reduced cofactors nicotinamide adenine dinucleotide (NADH) and FADH2 to molecular oxygen. Movement of hydrogen ions back into the mitochondrion via ATP synthase drives the synthesis of ATP.
PROTEIN PHOSPHATASE 1: An enzyme that will hydrolyze phosphate groups from target proteins such as glycogen synthase, phosphorylase, phosphorylase kinase, and the phosphorylated form of inhibitor 1. The phosphorylated inhibitor 1 is a substrate that binds well but is hydrolyzed slowly. While bound to protein phosphatase 1, the phosphorylated inhibitor 1 serves as an inhibitor of the enzyme.
DISCUSSION
The liver is a highly specialized organ that plays a central role in whole body glucose metabolism. During periods of increased glucose availability, the liver increases uptake, storage, and utilization of glucose. By contrast, when exogenous glucose availability declines (eg, during an overnight fast), the liver increases glucose production, thereby helping to maintain blood glucose levels. The liver uses 2 mechanisms for endogenous glucose production, the mobilization of intracellular glycogen (glycogenolysis) and the synthesis of glucose from noncarbohydrate precursors (gluconeogenesis; Figure 26-1). These pathways converge at glucose 6-phosphate. The latter is hydrolyzed to free glucose by glucose-6-phosphatase, the enzyme unique to gluconeogenic tissues, such as the liver. Once generated, glucose passes down its concentration gradient (eg, from the cytosol of the liver cell to the blood during periods of decreased blood glucose levels), via glucose transporter isoform 2 (GLUT 2).
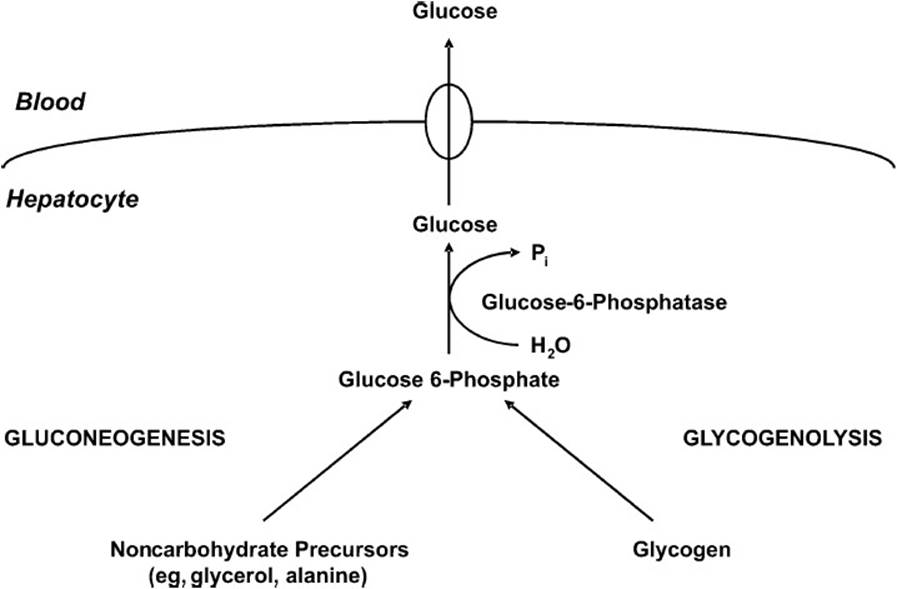
Figure 26-1. Schematic diagram showing events that will lead to the export of glucose from the liver cell during times of low blood glucose.
During periods of increased glucose availability (eg, postprandially), glucose in excess of the energetic demands of the organism is stored as glycogen (the storage form of glucose in mammals). Although found within the cytosol of virtually every cell, glycogen is primarily concentrated in muscle (cardiac and skeletal) and liver. The purpose of glycogen synthesis (glycogenesis) is to anticipate subsequent periods of decreased glucose availability (eg, overnight fast). During the latter period of time, glycogen will be mobilized as a readily available source of glucose. In the case of muscle, glycogen is selfishly used as an energy source by the myocyte alone. By contrast, liver glycogen will be mobilized to help maintain blood glucose levels. The biochemical pathways of glycogenesis and glycogenolysis are illustrated in Figure 26-2.
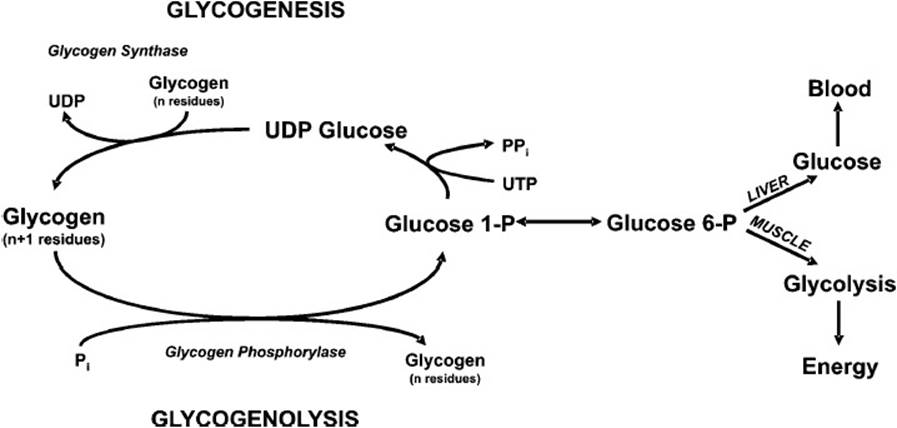
Figure 26-2. The biochemical pathways of the synthesis of glycogen (glycogenesis) and its breakdown to glucose 6-phosphate (glycogenolysis).
As glycogenesis is the reciprocal pathway to glycogenolysis, gluconeogenesis is the reciprocal pathway to glycolysis. Glycolysis, the “lysis” of glucose to 2 pyruvate molecules, is a ubiquitous metabolic pathway, whereas gluconeogenesis occurs in only a select number of tissues, including the liver. During periods of increased glucose availability, flux through the glycolytic pathway increases, thereby utilizing this readily available fuel source. By contrast, during periods of decreased glucose availability, rates of gluconeogenesis increase, in an attempt to maintain blood glucose levels. The sources of carbon for gluconeogenesis depend on the given metabolic situation (eg, exercise, starvation, diabetes mellitus), and include glycerol, amino acids, and lactate. Although the majority of fatty acid–derived carbon cannot be used for the net synthesis of glucose, fatty acid metabolism plays a central role in gluconeogenesis. Gluconeogenesis is an energetically demanding process, which is driven by the β-oxidation of fatty acids. When the rate of acetyl-CoA generation through fatty acid β-oxidation exceeds the rate of acetyl-CoA oxidation via the Krebs cycle and oxidative phosphorylation (ox phos), acetyl-CoA accumulates. This acetyl-CoA is shunted into the pathway of ketone body synthesis (ketogenesis), allowing continued β-oxidation of fatty acids and therefore maintenance of high rates of gluconeogenesis. The interplay between glycolysis, gluconeogenesis, β-oxidation, and ketogenesis is illustrated in Figure 26-3.
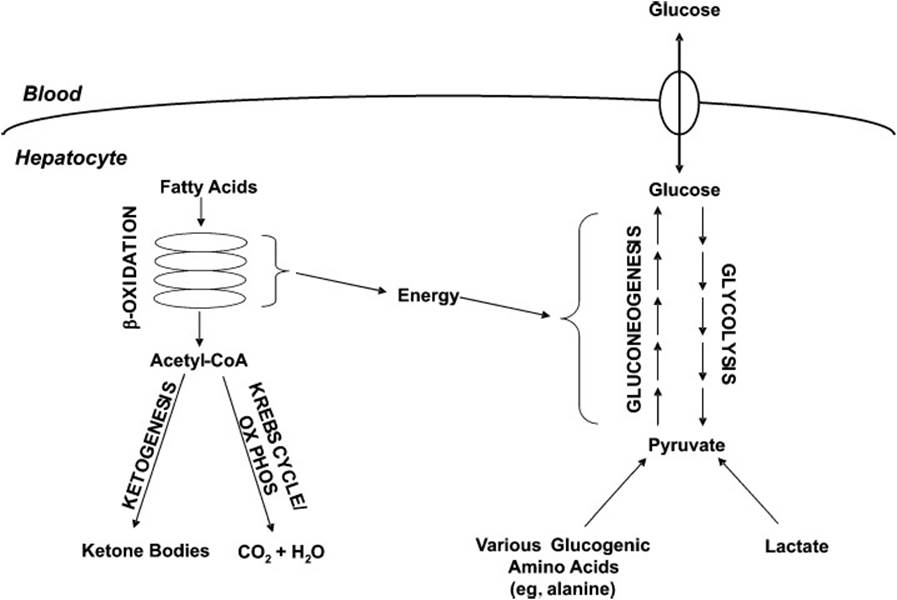
Figure 26-3. Schematic diagram of the interplay of fatty acid breakdown and ketone body formation with the synthesis (gluconeogenesis) and degradation of glucose (glycolysis). The β-oxidation of fatty acids provides the energy that drives the formation of glucose.
Flux of carbon through the pathways of hepatic glucose metabolism described above is strongly influenced by the hormones insulin, glucagon, and epinephrine. Insulin is secreted from the β-cells of pancreatic islets during periods of increased glucose availability. This peptide hormone helps to lower blood glucose back within the normal range by stimulating glycogenesis and glycolysis, and simultaneously inhibiting glycogenolysis and gluconeogenesis. The effects of insulin on hepatic glucose metabolism are mediated in large part by the enzyme protein phosphatase 1 (PP1). For a more detailed discussion of the mechanism by which PP1 affects glucose metabolism, see case 22.
When blood glucose levels begin to decline (eg, during an overnight fast), so too does insulin secretion. By contrast, the secretion of glucagon from α-cells of pancreatic islets is stimulated. The latter targets primarily hepatic glucose metabolism, increasing glucose production (via gluconeogenesis and glycogenolysis), and decreasing glucose utilization. Glucagon acts in larger part by reversing the effects of insulin-mediated PP1 activation. On binding to its cell surface receptor, glucagon increases the activity of protein kinase A (PKA), which phosphorylates many of the proteins/enzymes that PP1 dephosphorylates. For a detailed discussion, of the mechanism by which PKA acts, see case 22.
Like glucagon, epinephrine secretion increases during periods of decreased glucose availability. Binding of epinephrine to β-adrenergic receptors on the surface of the hepatocyte results in activation of PKA, thereby increasing further hepatic glucose production through glycogenolysis and gluconeogenesis (Figure 26-4). Epinephrine is also able to bind to a second receptor on the surface of the hepatocyte, the β-adrenergic receptor, causing elevation of intracellular Ca2+ levels. The latter allosterically activates a kinase called phosphorylase kinase, which, in turn, augments activation of glycogenolysis.
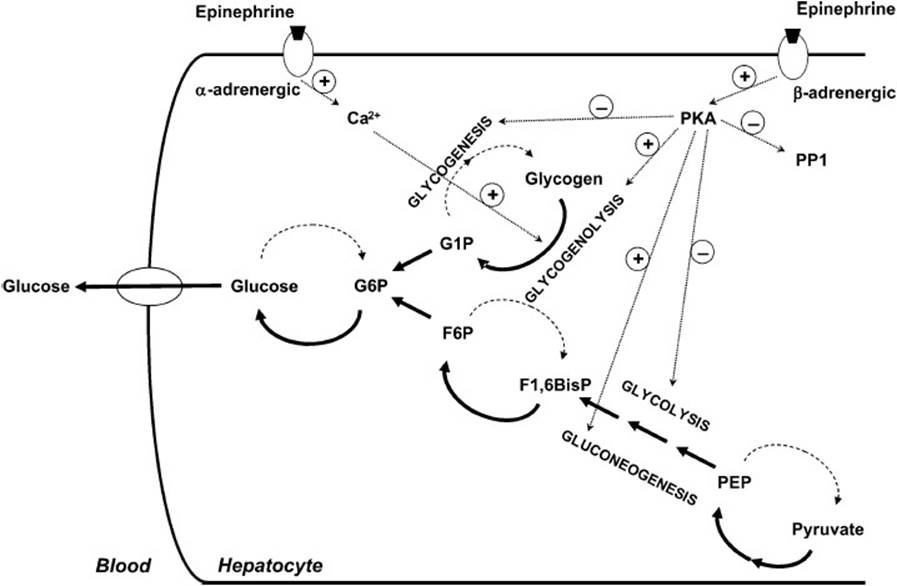
Figure 26-4. Schematic diagram showing how epinephrine leads to the breakdown of glycogen, the synthesis of glucose and export of glucose to the bloodstream. Epinephrine binds to both α- and β-adrenergic receptors on the plasma membrane of hepatocytes, leading to increased release of Ca2+ and activation of adenylate cyclase and protein kinase A.
Abnormalities in the above described glucose homeostatic mechanisms arise during diabetes mellitus. Two major forms of diabetes mellitus exist, insulin-dependent (type 1) and insulin-independent (type 2) diabetes. Type 1 diabetes is caused by a severe lack or complete absence of insulin. Also known as early onset diabetes, this disease is often caused by an autoimmune destruction of pancreatic β-cells. Lack of insulin, in the face of elevated glucagon and epinephrine, leads to high rates of hepatic glucose output, being driven by β-oxidation of fatty acids. The latter results in an excessive production of ketone bodies and subsequent ketoacidosis. Treatment of type 1 diabetes involves regular monitoring of blood glucose levels and insulin administration as required. Insulin therapy associated with an evening meal will lower blood glucose levels. The latter triggers release of the counter-regulatory hormones glucagon and epinephrine, thereby stimulating hepatic glucose production. This inadvertently results in elevated glucose levels in the morning (the Somogyi effect). By contrast to type 1 diabetes, type 2 diabetes is caused by insulin resistance in the face of insulin sufficiency. Thus, the disease is characterized by both hyperglycemia and hyperinsulinemia. Ketoacidosis is a less common complication of type 2 diabetes.
COMPREHENSION QUESTIONS
26.1 A 27-year-old man has been rushed to the emergency department following his sudden collapse and entry into a state of unconsciousness. Examination of personal belongings revealed the patient is an insulin-dependent diabetic. A rapid decline in which of the following humoral factors likely triggered the sudden collapse of the patient?
A. Insulin
B. Glucagon
C. Fatty acids
D. Glucose
E. Triglyceride
26.2 Which of the following is the least likely to contribute to the hyperglycemia associated with uncontrolled type 1 diabetes?
A. Decreased skeletal muscle glucose uptake
B. Decreased adipose lipogenesis
C. Increased adipose lipolysis
D. Increased hepatic gluconeogenesis
E. Increased skeletal muscle glycogenolysis
26.3 Which of the following changes in hepatic metabolism best explains the increased incidence of ketoacidosis observed in type 1 diabetes?
A. Increased glucose uptake
B. Increased protein synthesis
C. Increased lipoprotein synthesis
D. Increased β-oxidation
E. Increased glycogen breakdown
ANSWERS
26.1 D. Glucose is the primary source of energy for the central nervous system. A sudden decrease in circulating glucose levels will therefore impair ATP generation, in turn impeding cognitive function. If hypoglycemia persists, then the patient will slip into a coma and eventually die. This unfortunately common complication in people with type 1 diabetes is a consequence of oversupplementation with insulin. By contrast, a sudden decrease in circulating insulin, glucagon, fatty acids, or triglyceride would have little immediate effect on cognitive function.
26.2 E. Unlike the liver, skeletal muscle cannot export glucose into the circulation. Once glucose enters the myocyte, it is destined for use by that cell. Thus, intramyocellular glycogen is used as a fuel source by skeletal muscle and therefore cannot contribute to the hyperglycemia observed in uncontrolled type I diabetes. In contrast decreased insulin-mediated glucose utilization by skeletal muscle and adipose will contribute to hyperglycemia, as will decreased insulin-mediated suppression of hepatic glucose output. Decreased insulin-mediated suppression of lipolysis will indirectly contribute to hyperglycemia, by providing alterative, nonglucose fuels (fatty acids and ketone bodies) for organs such as skeletal muscle and the liver.
26.3 D. Decreased circulating insulin signals a need to increase hepatic glucose production. This is an energetically demanding process, driven by β-oxidation of fatty acids. However, acetyl-CoA, the major end product of β-oxidation, cannot be used for glucose production. Instead, acetyl-CoA is shunted into the pathway of ketone body synthesis (ketogenesis). By contrast, carbon from intrahepatic glycogen will contribute less to ketone body synthesis in uncontrolled type 1 diabetes mellitus. Net glucose uptake, protein synthesis, and lipoprotein synthesis are decreased, as opposed to increased, during uncontrolled type 1 diabetes.
BIOCHEMISTRY PEARLS
![]() The liver uses 2 mechanisms for endogenous glucose production, the mobilization of intracellular glycogen (glycogenolysis) and the synthesis of glucose from noncarbohydrate precursors (gluconeogenesis).
The liver uses 2 mechanisms for endogenous glucose production, the mobilization of intracellular glycogen (glycogenolysis) and the synthesis of glucose from noncarbohydrate precursors (gluconeogenesis).
![]() Flux of carbon through the pathways of hepatic glucose metabolism described above is strongly influenced by the hormones insulin, glucagon, and epinephrine.
Flux of carbon through the pathways of hepatic glucose metabolism described above is strongly influenced by the hormones insulin, glucagon, and epinephrine.
![]() Lack of insulin, in the face of elevated glucagon and epinephrine, leads to high rates of hepatic glucose output, driven by β-oxidation of fatty acids. The latter results in an excessive production of ketone bodies and subsequent ketoacidosis.
Lack of insulin, in the face of elevated glucagon and epinephrine, leads to high rates of hepatic glucose output, driven by β-oxidation of fatty acids. The latter results in an excessive production of ketone bodies and subsequent ketoacidosis.
REFERENCES
Cohen P. Dissection of the protein phosphorylation cascades involved in insulin and growth factor action. Biochem Soc Trans. 1993;21:555.
Newsholme EA, Leech AR. Biochemistry for the Medical Sciences. New York: Wiley; 1983.