Case Files Biochemistry, 3rd Edition (2015)
SECTION II. Clinical Cases
CASE 27
A 51-year-old man presents to the emergency department with chest pain. He states that he has had chest discomfort or pressure intermittently over the last year especially with increased activity. He describes the chest pain as a pressure behind his breastbone that spreads to the left side of his neck. Unlike previous episodes, he was lying down, watching television. The chest pain lasted approximately 15 minutes then subsided on its own. He also noticed that he was nauseated and sweating during the pain episode. He has no medical problems that he is aware of and has not been to a physician for several years. On examination, he is in no acute distress with normal vital signs. His lungs were clear to auscultation bilaterally, and his heart had a regular rate and rhythm with no murmurs. Electrocardiography (ECG) revealed ST segment elevation and peaked T waves in leads II, III, and aVF. Serum troponin I and T levels are elevated.
![]() What is the most likely diagnosis?
What is the most likely diagnosis?
![]() What biochemical shuttle may be active to produce more adenosine triphosphate (ATP) per glucose molecule?
What biochemical shuttle may be active to produce more adenosine triphosphate (ATP) per glucose molecule?
ANSWERS TO CASE 27:
Myocardial Infarction
Summary: A 51-year-old man with a history of chest pain with exertion presents with retrosternal chest pressure that radiates to the neck. He has nausea and diaphoresis while at rest. The patient has ST segment elevation and peaked T waves in the inferior ECG leads. The troponin I and T levels are elevated.
• Likely diagnosis: Acute myocardial infarction.
• Biochemical shuttle: The malate-aspartate shuttle is primarily seen in the heart, liver, and kidney. This shuttle requires cytosolic and mitochondrial forms of malate dehydrogenase and glutamate-oxaloacetate transaminase and 2 antiporters, the malate-α-ketoglutarate antiporter and the glutamate-aspartate antiporter, which are both localized in the mitochondrial inner membrane. In this shuttle cytosolic nicotinamide adenine dinucleotide (NADH) is oxidized to regenerate cytosolic NAD+ by reducing oxaloacetate to malate by cytosolic malate dehydrogenase.
CLINICAL CORRELATION
The most common cause of death of Americans is coronary heart disease. The patient’s symptoms in this case are very typical of myocardial infarction, that is, chest pressure or chest pain, often radiating to the neck or to the left arm. The pain is usually described as deep and “squeezing chest pain.” Cardiac muscle is perfused by coronary arteries with very little redundant or shared circulation; thus, occlusion of one coronary artery usually leads to ischemia or necrosis of the corresponding cardiac muscle. Laboratory confirmation of myocardial infarction (death of cardiac muscle) includes ECG showing elevation of the ST segment, an increase in cardiac enzymes, or both. When there is insufficient oxygen available for the cardiac muscle, then the glycolytic pathway must be used, which leads to a very small amount of ATP per glucose molecule. The malate-aspartate shuttle can offer 2 or 3 more times the ATP by oxidizing NADH to regenerate cytosolic NAD+ by reducing oxaloacetate to malate by cytosolic malate dehydrogenase.
APPROACH TO:
Glycolysis and the Malate-Aspartate Shuttle
OBJECTIVES
1. Be familiar with glycolysis.
2. Know the role of glycerol 3-phosphate and the malate-aspartate shuttle.
3. Be aware of the role of mitochondria in glycolysis.
DEFINITIONS
MYOCARDIAL INFARCTION: An area of heart muscle is inadequately perfused as a result of cardiac vessel occlusion resulting in ischemia, cell death, and loss of cell constituents including enzymes into the circulation. Electrocardiographic changes occur as a result of damaged heart tissue.
ANGINA: Transient disruption of adequate blood flow to a portion of heart muscle leading to pain and a temporary shift to anaerobic glycolysis producing pyruvate and lactate that are released into the circulation.
AEROBIC GLYCOLYSIS: Metabolism of glucose to pyruvate. Pyruvate in the presence of sufficient oxygen can be metabolized to CO2 via the tricarboxylic acid cycle in the mitochondrion-producing NADH and FADH2, which contribute electrons through the electron transfer chain to molecular oxygen producing H2O and ATP.
ANAEROBIC GLYCOLYSIS: Metabolism of glucose to lactate in the absence of sufficient oxygen. When oxygen is lacking, pyruvate is converted to lactate, and no further oxidative pathway is available.
ELECTRON SHUTTLES: Enzymatic processes whereby electrons from NADH can be transferred across the mitochondrial barrier. The glycerol 3-phosphate shuttle uses the reduction of dihydroxyacetone phosphate to glycerol 3-phosphate and reoxidation to transfer electrons from cytosolic NADH to coenzyme Q in the electron transport chain. The malate-aspartate shuttle uses malate and aspartate in a 2-member transfer exchange to transfer electrons from cytosolic NADH to mitochondrial NADH (Figures 27-1 and 27-2).
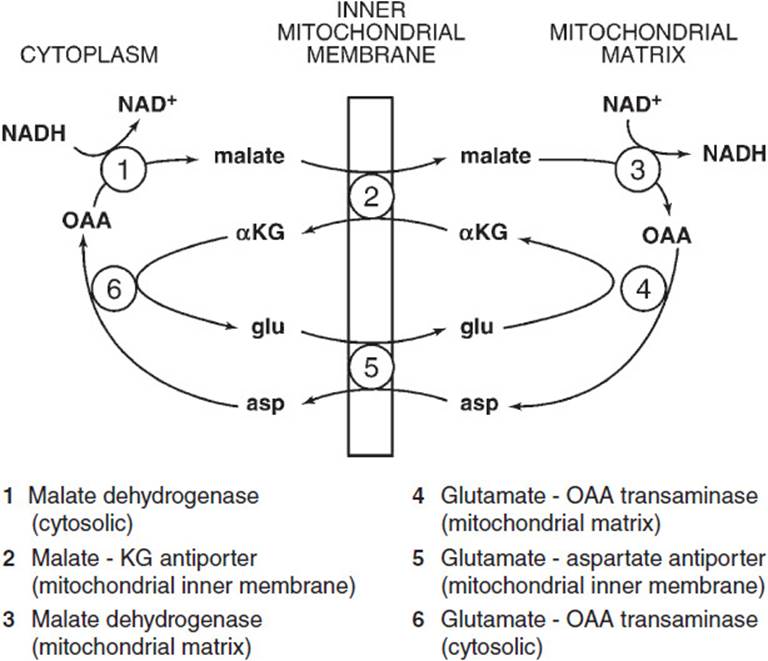
Figure 27-1. Malate-aspartate shuttle.
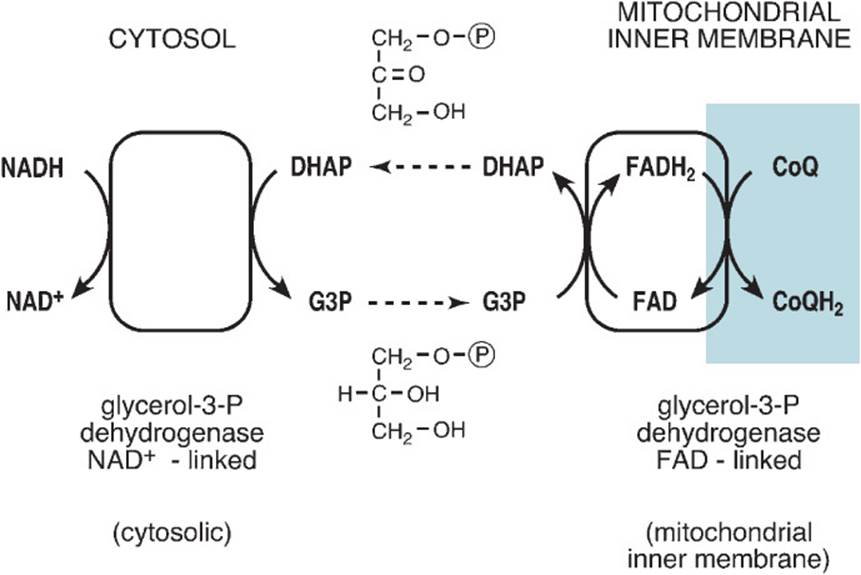
Figure 27-2. Glycerol 3-phosphate shuttle.
DISCUSSION
Myocardial infarction arises when perfusion of cardiac muscle is inadequate, resulting in insufficient oxygen delivery to that portion of cardiac muscle. This causes the affected muscle to rely on anaerobic metabolism for its energy supply with concomitant production of lactic acid. Even transient ischemia can lead to changes in muscle tissue, but prolonged ischemia leads to breakdown of muscle cells and release of cellular proteins such as creatine kinase, lactic acid dehydrogenase, and troponin I. Reperfusion by thrombolytic treatment or mechanical means can restore oxygen levels and return the metabolic processes to aerobic metabolism. A secondary consequence of reperfusion is reperfusion injury in which the highly reduced state of injured cells meets increased oxygen concentration and produces reactive oxygen radicals. Most notable of these is the hydroxyl radical (OH•), which attacks tissue components such as lipids and protein sulfhydryl groups. Myocardial infarction causes changes in the pathways of energy generation triggered by oxygen insufficiency in the affected heart muscle.
The glycolytic pathway for all cells under conditions of adequate tissue oxygenation is shown in Figure 27-3. Two molecules of ATP are required to initiate the pathway—one at the hexokinase step to phosphorylate glucose to glucose 6-phosphate and a second to phosphorylate fructose 6-phosphate to fructose 1,6-bisphosphate. Cleavage of fructose 1,6-bisphosphate by aldolase yields 2 triose phosphates, glyceraldehyde 3-phosphate, and dihydroxyacetone phosphate, which is isomerized to glyceraldehyde 3-phosphate by triose phosphate isomerase. The oxidation of the aldehyde group to the acid level through the reduction of NAD+ to NADH with the concomitant binding of inorganic phosphate to produce 1,3-bisphosphoglycerate is catalyzed by 3-phosphoglyceraldehyde dehydrogenase. This step requires NAD+ regeneration to continue. Inorganic phosphate is present in tissues at such a high concentration (typically 25 mM) as not to be limiting. The first substrate level phosphorylation occurs next, wherein phosphoglycerate kinase catalyzes the transfer of the acyl phosphate group of 1,3-bisphosphoglycerate to adenosine diphosphate (ADP), yielding ATP and 3-phosphoglycerate. Phosphoglucomutase shifts the phosphate of 3-phosphoglycerate to carbon position 2 producing 2-phosphoglycerate. Enolase catalyzes the removal of the elements of water between carbon positions 2 and 3 to produce phosphoenolpyruvate. Pyruvate kinase then catalyzes the second substrate level phosphorylation of ADP to produce ATP and pyruvate, the end product of aerobic glycolysis. Because fructose 1,6-bisphosphate is cleaved to 2 triose phosphate moieties and each triose phosphate produces 2 ATP molecules, the total produced by substrate level phosphorylation is 4 ATP molecules. But 2 ATP molecules are consumed in the activation of hexose leaving 2 ATP molecules as the net gain from glycolytic substrate level phosphorylation.
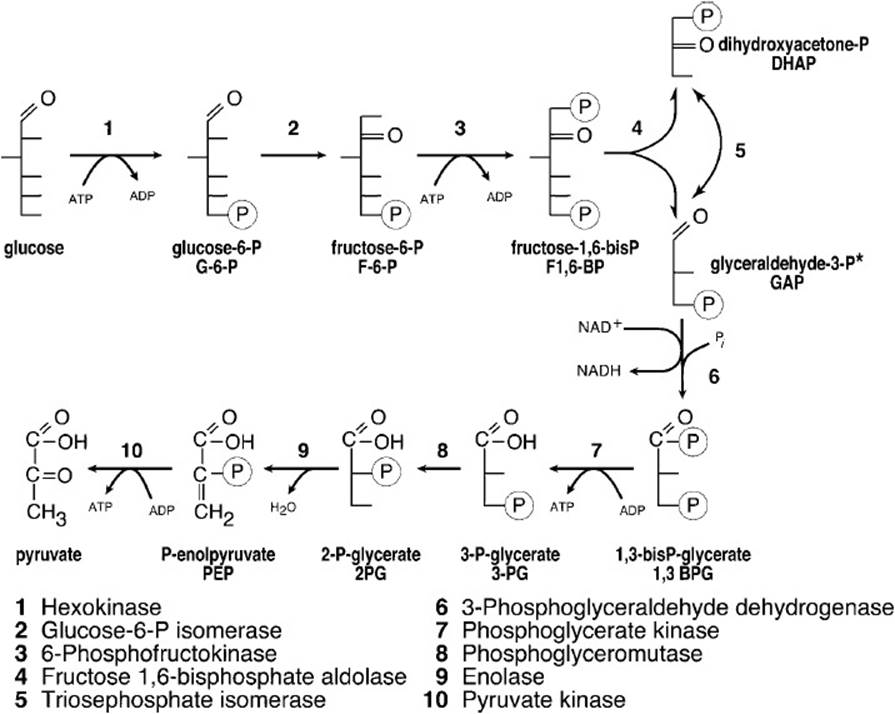
Figure 27-3. Glycolytic pathway.
*From glyceraldehyde 3-phosphate onward, 2,3-carbon molecules/glucose proceed through the pathway requiring a total of 2NAD+ and 4ADP producing a total of 4ATP, 2NADH, and 2H2O.
As noted above, however, NAD+ must be regenerated from the NADH produced or the glycolytic cycle would cease. Under aerobic conditions regeneration of cytosolic NAD+ from cytosolic NADH is accomplished by transferring electrons across the mitochondrial membrane barrier to the electron transfer chain where the electrons are transferred to oxygen. There are 2 different shuttle mechanisms whereby this transfer of electrons across the membrane to regenerate cytosolic NAD+ can be accomplished: the glycerol 3-phosphate shuttle and the malate-aspartate shuttle.
The glycerol 3-phosphate shuttle functions primarily in skeletal muscle and brain (Figure 27-2). The shuttle takes advantage of the fact that the enzyme glycerol-3-phosphate dehydrogenase exists in 2 forms, a cytosolic form that uses NAD+ as cofactor and a mitochondrial FAD-linked form. Cytosolic glycerol-3-phosphate dehydrogenase uses electrons from cytosolic NADH to reduce the glycolytic intermediate dihydroxyacetone phosphate to glycerol 3-phosphate, thereby regenerating cytosolic NAD+. The newly formed glycerol 3-phosphate is released from the cytosolic form of the enzyme and crosses to and is bound to the mitochondrial FAD-linked glycerol-3-phosphate dehydrogenase, which is bound to the cytosolic side of the mitochondrial inner membrane. There the mitochondrial glycerol-3-phosphate dehydrogenase reoxidizes glycerol 3-phosphate to dihydroxyacetone phosphate (preserving mass balance), reducing its FAD cofactor to FADH2. Electrons are then passed to coenzyme Q of the electron transport chain and on to oxygen generating 2 additional ATP molecules per electron pair and therefore per glycerol 3-phosphate.
However, the malate-aspartate shuttle functions primarily in the heart, liver, and kidney (Figure 27-3). This shuttle requires cytosolic and mitochondrial forms of malate dehydrogenase and glutamate-oxaloacetate transaminase and 2 antiporters, the malate-α-ketoglutarate antiporter and the glutamate-aspartate antiporter, which are both localized in the mitochondrial inner membrane. In this shuttle cytosolic NADH is oxidized to regenerate cytosolic NAD+ by reducing oxaloacetate to malate by cytosolic malate dehydrogenase. Malate is transported into the mitochondrial matrix while α-ketoglutarate is transported out by the malate-α-ketoglutarate antiporter, seemingly a mass imbalance. Next malate is oxidized back to oxaloacetate producing NADH from NAD+ in the mitochondrial matrix by mitochondrial malate dehydrogenase. Oxaloacetate cannot be transported per se across the mitochondrial membrane. It is instead transaminated to aspartate from the NH3 donor glutamate by mitochondrial glutamate-oxaloacetate transaminase. Aspartate is transported out of the matrix whereas glutamate is transported in by the glutamate-aspartate antiporter in the mitochondrial membrane, obviating the apparent mass unbalance noted above. The last step of the shuttle is catalyzed by cytosolic glutamate-oxaloacetate transaminase regenerating cytosolic oxaloacetate from aspartate and cytosolic glutamate from α-ketoglutarate both of which were earlier transported in opposing directions by the malate-α-ketoglutarate antiporter. The net effect of this shuttle is to transport electrons from cytosolic NADH to mitochondrial NAD+. Therefore, those electrons can be presented by the newly formed NADH to electron transport system complex I, thereby producing 3 ATP molecules by oxidative phosphorylation. Note that depending on which shuttle is used (ie, which tissue is catalyzing glycolysis) either 2 or 3 extra ATP molecules are produced by oxidative phosphorylation per triose phosphate going through the latter steps of glycolysis.
In nonaerobic glycolysis, as in the case when a tissue is subjected to an ischemic episode (ie, myocardial infarction), neither the extra ATP molecule produced by the shuttle nor the ATP molecules produced by normal passage of electrons through the electron transport chain are produced because of oxygen insufficiency. Therefore, glycolysis must increase in rate to meet the energy demand. In damaged tissue this increased rate is compromised. Moreover, the shuttle mechanisms to regenerate NAD+ from NADH formed by glycolysis are unavailable, as shown in Figure 27-4. Glycolysis under ischemic conditions satisfies the requirement for NAD+ by reducing pyruvate, the glycolytic end product under normative conditions, to lactate with the reducing equivalents of NADH.
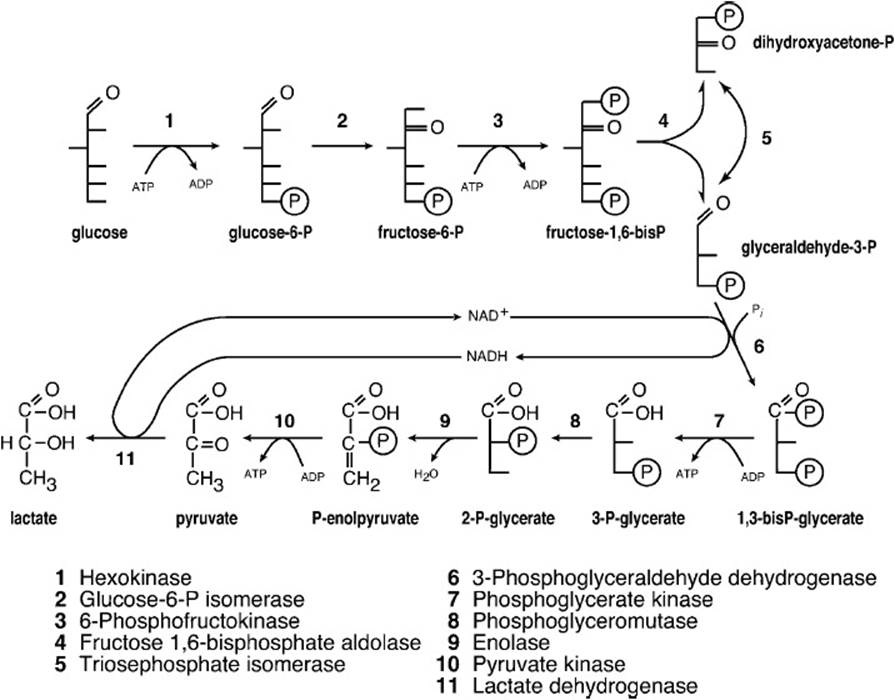
Figure 27-4. Glycolytic pathway under conditions of insufficient oxygen.
The new end product lactate accumulates in muscle cells under ischemic conditions and damages cell walls with its low pH causing rupture and loss of cell contents such as myoglobin and troponin I. These compounds as well as other end products combine to cause increased cell rupture and pain.
Reopening vasculature by reperfusion as rapidly as possible is a first step in treatment. Thrombolysis within an hour after infarction gives best results. Supportive measures, regulation of heart rate and pressure are required following the infarction to allow recovery from the ischemic episode and repair of tissue damage. Nutritional monitoring is required both for tissue repair and prevention of recurrence.
COMPREHENSION QUESTIONS
27.1 Following an episode of retrosternal chest pressure with radiation to the neck and associated nausea and diaphoresis while at rest, a middle-aged man was diagnosed with unstable angina and possibly myocardial infarction. Subsequent laboratory findings of increased serum levels of troponin I and cardiac enzymes were consistent with the diagnosis.
If the patient described above did indeed have angina, which of the following change(s) in metabolism in the affected area would occur?
A. Increased oxidative phosphorylation
B. Increased rate of fatty acid oxidation
C. Increased conversion of pyruvate to acetyl-CoA
D. Increased formation of lactate
E. Increased use of ketone bodies
27.2 In the case described in question 27.1, an ischemic episode very likely occurred. What changes in glucose metabolism would be observed?
A. The overall rate of glucose utilization would decrease.
B. Pyruvate kinase is allosterically inhibited.
C. The rate of ATP production in the cytosol increases.
D. NADH is reoxidized to NAD+ via the glycerol 3-phosphate shuttle.
27.3 If the patient described in question 27.1 does indeed have angina, his is a focal lesion wherein only one portion of heart muscle is affected while other portions still receive adequate oxygen, enabling the malate-aspartate shuttle to function. Which of the following is involved in this shuttle to transfer electrons across the mitochondrial barrier?
A. Malate-aspartate antiporter
B. Malate-α-ketoglutarate antiporter
C. Glutamate-α-ketoglutarate antiporter
D. Aspartate-α-ketoglutarate symporter
E. Malate-glutamate symporter
ANSWERS
27.1 D. In this case the problem is insufficient oxygen reaches an area of cardiac muscle. In the absence of sufficient oxygen, the use of the electron transfer chain to generate ATP from ADP is severely compromised, and therefore all products feeding into that chain accumulate reducing the formation of tricarboxylic acid products. Fatty acid oxidation, formation of acetyl-CoA from pyruvate oxidation of ketone bodies, and oxidative phosphorylation are all decreased. In glycolysis under reduced oxygen conditions, one pyruvate is converted to lactate to reoxidize NADH to NAD+ to meet the requirement for glycolysis to continue. Therefore, there is increased formation of lactate.
27.2 C. The rates of both glucose utilization and ATP generation by glycolysis increase to compensate for the absence of sufficient ATP production from oxidative phosphorylation as a result of oxygen deprivation. Under these circumstances, ATP is used as rapidly as it is made so it is not present in sufficient concentration to inhibit pyruvate kinase. Furthermore, fructose 1,6-bisphosphate tends to stimulate pyruvate kinase activity.
27.3 B. The malate-α-ketoglutarate antiporter carries malate from the cytosol, where it is oxidized to oxaloacetate by mitochondrial malate dehydrogenase, forming NADH. In order for the malate-α-ketoglutarate antiporter to function, a molecule of α-ketoglutarate must be transported from the mitochondrial matrix to the cytosol. The only other transporter involved in this shuttle mechanism is the aspartate-glutamate antiporter. None of the other suggested transporters is required in this shuttle mechanism.
BIOCHEMISTRY PEARLS
![]() Coronary heart disease is the most common cause of mortality in Americans.
Coronary heart disease is the most common cause of mortality in Americans.
![]() Myocardial infarction is suspected with typical chest pain, ECG findings of ST segment elevation, and increased cardiac enzymes.
Myocardial infarction is suspected with typical chest pain, ECG findings of ST segment elevation, and increased cardiac enzymes.
![]() When oxygen is unavailable, the anaerobic glycolytic pathway is used.
When oxygen is unavailable, the anaerobic glycolytic pathway is used.
![]() Two shuttles allow for more ATP to be generated under aerobic conditions: the glycerol 3-phosphate shuttle, which functions primarily in skeletal muscle and brain, and the malate-aspartate shuttle, primarily in the heart, liver, and kidney.
Two shuttles allow for more ATP to be generated under aerobic conditions: the glycerol 3-phosphate shuttle, which functions primarily in skeletal muscle and brain, and the malate-aspartate shuttle, primarily in the heart, liver, and kidney.
REFERENCES
Devlin TM, ed. Textbook of Biochemistry with Clinical Correlations. 7th ed. New York: Wiley-Liss; 2010.
Longo D, Fauci AS, Kaspar D, et al, eds. Harrison’s Principles of Internal Medicine. 18th ed. New York: McGraw-Hill; 2011.