Case Files Biochemistry, 3rd Edition (2015)
SECTION II. Clinical Cases
CASE 33
A 28-year-old Lebanese man presents to your office with complaints of fatigue and difficulty catching his breath with his normal exercise routine since recently visiting his wife’s family. He states he is otherwise healthy. He denies fever/chills/nausea/vomiting/diarrhea. He reports no sick contacts or travel other than to his in-law’s house where he ate their famous fava bean soup. Upon further questioning, he reports that his urine has become much more concentrated and dark despite adequate fluid intake. On examination, he is an ill appearing male who appears fatigued/pale. His sclera are icteric and is conjunctiva are pale. His heart rate is slightly tachycardic at 110 beats/minute and his capillary refill is delayed. The remainder of the examination is unremarkable. Urinalysis is collected and revealed very dark urine. His hematocrit is 21% (normal: 40%–49%).
![]() What is the most likely diagnosis?
What is the most likely diagnosis?
![]() What is the inheritance pattern of this disorder?
What is the inheritance pattern of this disorder?
ANSWERS TO CASE 33:
Glucose 6-Phosphate Dehydrogenase Deficiency
Summary: A 28-year-old Lebanese man with new onset fatigue, shortness of breath, jaundice, and anemia following a recent travel to in-law’s home where he ate fava bean soup.
• Likely diagnosis: Acute hemolytic anemia caused by underlying G6PD deficiency.
• Inheritance pattern: X-linked (predominantly seen in males)
CLINICAL CORRELATION:
Glucose 6-phosphate dehydrogenase (G6PDH) is an X-linked genetic condition that causes instability to the red cell membrane leading to rupture. Fava beans precipitate acute hemolytic anemia in individuals with low levels of G6PDH and, thus, lowered levels of NADPH, reducing equivalents to suppress oxidative damage. Oxidative radical formation damages and compromises the integrity of the red blood cell membrane, leading to hemolysis (hemolytic anemia) and the clinical symptoms described in the case. In addition to Fava bean ingestion, other common causes of glucose-6-dehydrogenase deficiency hemolytic anemia include drugs (primaquine), chemicals (moth balls, aniline dyes, henna compounds), infection (Escherichia coli, Salmonella), and diabetic ketoacidosis (DKA). Patients confirmed to have this deficiency must be aware of these triggers.
APPROACH TO:
Glucose 6-Phosphate Dehydrogenase Deficiency
OBJECTIVES
1. Describe how NADPH is produced in the red blood cell and its role in maintaining erythrocyte integrity.
2. Explain how dietary challenges can lead to hemolysis in individuals with low G6PDH activity.
3. Recognize the inheritance pattern and population distribution of G6PDH deficiencies.
DEFINITIONS
ACUTE HEMOLYTIC ANEMIA: A pathologic condition in which there is a sudden drop in the number of circulating erythrocytes precipitated by an acute challenge which overwhelms the ability of the erythrocyte to defend against an oxidative attack on the lipids of the erythrocyte membrane resulting in membrane rupture.
GLUCOSE 6-PHOSPHATE DEHYDROGENASE (G6PDH): The enzyme catalyzing the rate limiting step of the hexose monophosphate shunt which provides reducing equivalents in the form of NADPH required to inactivate oxygen radicals attacking erythrocyte membrane lipids and protecting the erythrocyte membrane from rupture.
FAVA BEAN: Vicia faba, a bean of the species Fabaceae. It is native to the regions of Southwest Asia and North Africa and is cultivated widely around the world. Evidence indicates that the Fava bean had been cultivated in the Eastern Mediterranean since 6000 BC, if not earlier.
VICINE, CONVICINE, DIVICINE, AND ISOURAMIL: Compounds active in precipitating acute hemolytic anemia resulting from ingestion of fava beans by an individual with compromised G6PDH activity. Vicine and convicine are the naturally occurring glycosides while divicine and isouramil are the aglycones resulting from enzymatic removal of the glucoside moiety.
DISCUSSION
Hemolytic anemia is a condition in which erythrocytes are unable to transport sufficient oxygen to tissues throughout the body to meet the demands for energy generation through metabolism, usually due to premature destruction of red blood cells. The condition may be of either extrinsic or intrinsic origin. Defects in the structural properties of the red blood cell membrane and abnormalities in the proteins (eg, enzymes) within the erythrocyte are intrinsic causes of hemolytic anemia, while abnormalities in erythropoiesis, blood clots in the small vessels, certain infections and the “side effects” of certain therapeutic drugs constitute extrinsic causes of hemolytic anemia. Hemolytic anemias may also be described as chronic (eg, a defect in hemopoiesis) or chronic (eg, an infection or the side effect of a drug or dietary component). Hemolytic anemia arising as a consequence of a deficiency in G6PDH is described as intrinsic (enzyme deficiency) and acute because persons with this condition are free of symptoms until the erythrocytes come into contact with specific toxic drugs or dietary components. In this case, the event prompting the episode of acute hemolytic anemia is the ingestion of fava beans. This sensitivity to fava beans is well known in persons of Mediterranean, Middle Eastern, and African lineages, and it requires a genetic deficiency in G6PDH and a dietary issue, infection, or drug agent for expression of symptoms.
The genetic trait is carried on the X-chromosome at position g28. Because the trait is X-linked and recessive, the condition is more prevalent in males than in females, who would require 2 copies of the altered allele for expression of the condition. More than 400 variant alleles have been identified each leading to reduced G6PDH activity.
Role of Glucose 6-Phosphate Dehydrogenase in Maintenance of Erythrocyte Membrane Integrity
G6PDH is the first enzyme of 3 and the rate limiting step of the hexose monophosphate shunt (pentose shunt), which oxidizes glucose-6-phosphate to ribulose-5-phosphate, CO2, and 2 molecules of NADPH (shown schematically in Figure 33-1). The product of G6PDH activity is 1 NADPH and gluconolactone-6-P, which undergoes hydrolysis by the enzyme gluconolactone-6-P lactonase to form 6-phosphogluconate. A third enzyme, 6-phosphogluconate dehydrogenase, oxidizes 6-phosphogluconate to CO2, ribulose-5-P and the second molecule of NADPH. The Km values of the dehydrogenases for NADP+ and the Kí values for NADPH are quite close, such that the enzymes are highly regulated, essentially making an NADPH when one is used.
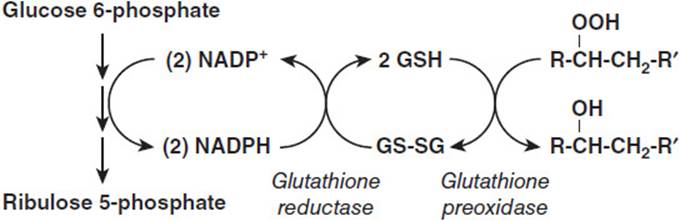
Figure 33-1. The glutathione reductase and peroxidase system. Glucose 6-phosphate dehydrogenase catalyzes the reaction represented by the first downward arrow in the 3-step conversion of glucose 6-phosphate to ribulose 5-phosphate. This conversion supplies the NADPH that reduces oxidized glutathione (GS-SG). The reduced glutathione (GSH) is used by glutathione peroxidase to reduce organic hydroperoxides to alcohols.
At the opposite end of the scheme shown in Figure 33-1 is illustrated the ultimate use of the NADPH reducing equivalents in the maintenance of red blood cell membrane integrity. Membrane fatty acyl residues present in the phospholipid bilayer can undergo oxidation to form fatty acyl hydroperoxides (R-HCH-CH2-R), which weaken the membrane, making it more susceptible to rupture as the red blood cell squeezes through the narrow diameters of the capillary beds in tissues. Reducing equivalents convert the hydroperoxide to a hydroxyl substituent, making the membrane less susceptible to rupture. This process requires the use of an intermediate carrier of reducing equivalents reduced glutathione and 2 additional enzymes, glutathione reductase, which transfers electrons from NADPH to oxidized glutathione, and glutathione peroxidase, which uses reduced glutathione to reduce the hydroperoxide moiety to the hydroxyl level generating oxidized glutathione as a second product.
If a male has one of the mutations expressing low activity of G6PDH, then he will be at risk for an episode of hemolytic anemia if he eats a meal containing fava beans. The symptoms develop because the deficient G6PDH activity is not able to prevent red blood cell membrane destruction (hemolysis) after eating the fava bean meal. After the fava bean digestion products are eliminated, normal erythropoieses will replace the missing red blood cells and the symptoms will be abated.
Role of Fava Beans in Prompting a Hemolytic Episode
The active components causing hemolytic episodes in persons with deficient G6PDH activity have been identified as the alkaloid β-glucosides vicine and convicine (Figure 33-2). These 2 compounds give rise by deglycosylation to the 2 aglycones divicine and isouramil, which also cause red blood cell hemolysis. In fact, divicine has been shown to be more toxic to erythrocytes than vicine and seems to be a direct cause of favism–erythrocyte hemolysis following exposure to these compounds. As seen in Figure 33-2, the structures of divicine and isouramil are redox active, allowing the reduction of the quinone form to the hydroxyl form and the reverse process as well. These compounds can be reduced with NADPH as the ultimate electron donor, and in individuals with deficient G6PDH activity the capacity to provide reducing equivalents is quickly overwhelmed. This makes possible the formation of superoxide anion (O2•-)and the hydroxyl radical (OH•) which can damage lipids in the membrane, predisposing the red blood cell to rupture by the physical pressures exerted on the erythrocyte as it traverses the narrows of the capillary network. The result is acute hemolytic anemia prompted by ingestion of fava beans.
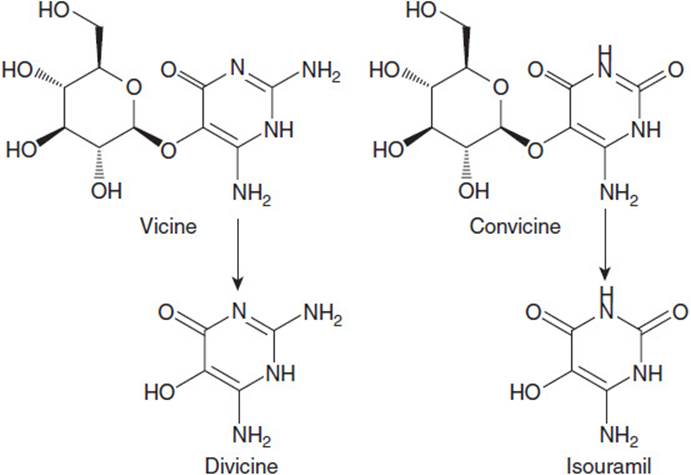
Figure 33-2. Structures of the alkaloid glucosides vicine and convicine present in fava beans. Each is metabolized to the corresponding aglycones, divicine, and isouramil.
COMPREHENSION QUESTIONS
33.1 A young otherwise healthy male patient complains of weakness and has a low erythrocyte count. You suspect a recent hemolytic crisis and check his erythrocytes and G6PDH levels. His erythrocyte count is down and his G6PDH is normal. You know that the pathway for protecting the erythrocyte membrane from damage is complex and that glutathione peroxidase is involved. Peroxidases use reducing equivalents produced by metabolism from glucose to reduce organic hydroperoxides to the hydroxyl level. Which of the following statements best describes the action of glutathione peroxidase?
A. Glutathione peroxidase reduces glutathione disulfide back to reduced glutathione.
B. Glutathione peroxidase produces 1 molecule of CO2 in its reaction cycle.
C. Glutathione peroxidase requires a selenium ion cofactor.
D. Glutathione peroxidase reduces organic hydroperoxides to organic hydroxyl compounds.
E. Glutathione peroxidase can use NADPH or NADH directly as a source of reducing equivalents.
33.2 A patient in your care has had a hemolytic crisis event and you have on your differential diagnosis notes that the event may indicate a dysfunction of G6PDH. You know that G6PDH in the red blood cell uses glucose 6-phosphate from the glycolytic pathway to form NADPH through a series of reactions. A person with deficient G6PDH would be expected to have all of the following characteristics except which of the following?
A. Increased proportion of glucose metabolism to pyruvate versus ribulose 5-phosphate than in normal
B. Decreased glutathione reductase activity
C. Decreased glutathione peroxidase activity
D. Increased reduction of organic hydroperoxides to hydroxyl products
E. Decreased production of gluconolactone-6-phosphate
33.3 During your third year, while on your hematology rounds, you are assigned a patient who has had an episode of hemolysis. Based on his recent consumption of fava bean soup and his Middle Eastern heritage you suspect a fava bean induced episode of active oxygen radicals may have caused the hemolysis. You know that fava beans contain divicine, isouramil, and aglycones that are redox active, permitting the transfer of electrons from one compound to another. When oxygen radical species are formed in individuals with deficient G6PDH activity, membrane lipids can undergo hydroperoxide formation. Which of the following statements is least accurate about erythrocytes in your patient?
A. Red blood cell membrane elasticity is reduced.
B. Red blood cells are more easily hemolyzed.
C. Red blood cells are more permeable.
D. Red blood cells easily traverse capillary beds.
E. Red blood cells are less able to reduce fatty acyl hydroperoxides to hydroxyl forms.
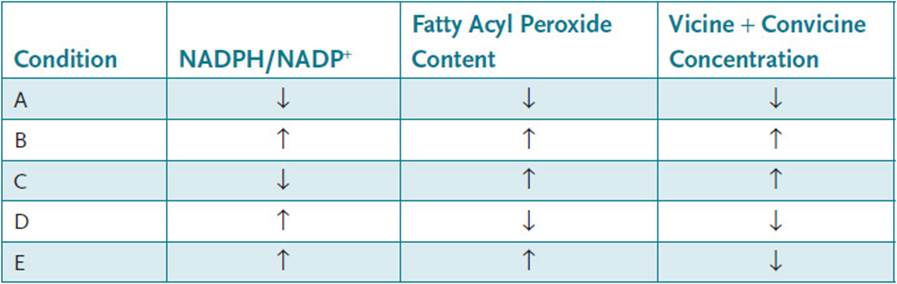
33.4 A young male patient with an episodic hemolytic crisis returns to your clinic several months later with another episode. He did not follow your advice to avoid certain foodstuffs. You now have another opportunity to pinpoint the precise cause of the malady. You know that G6PDH, the rate-limiting step of the hexose monophosphate shunt is regulated by the ratio of NADPH/NADP+ and that changes in binding efficiency could change the ability to protect the erythrocyte. Which condition would signal the enzyme to increase its activity?
ANSWERS
33.1 D. Glutathione peroxidase is the enzyme active in removing hydroperoxide products, resulting from reactive oxygen species attacks on membrane fatty acid substituents. Glutathione reductase reduces glutathione disulfide to reduced glutathione. 6-phosphogluconate dehydrogenase produces a molecule of CO2, an NADPH and ribulose-5-phosphates. Glutathione reductase requires a selenium cofactor. Glutathione peroxidase can only use reduced glutathione as an electron donor not NADPH and not NADH.
33.2 D. If G6PDH is deficient, then the pathway stops there and no reduced glutathione can be produced and, therefore, no removal of hydroperoxides occurs.
33.3 D. Erythrocytes with fatty acyl hydroperoxides have “stiff” membranes, which cannot squeeze through the smaller diameters of the capillaries. Membrane permeability is increased and the erythrocytes rupture rather than pass through the capillaries.
33.4 C. Elevated NADPH levels would inhibit G6PDH activity because [NADP+], a required substrate for the reaction, is decreased. Thus, choices B, D, and E can be eliminated. Decreased membrane fatty acyl hydroperoxide content would require less peroxidase activity and, therefore, less G6PDH activity, whereas elevated levels would tend to increase G6PDH activity if NADPH levels are reduced. Elevated vicine and convicine levels would increase membrane fatty acid hydroperoxide content, thus an increased need for peroxidase activity, and, therefore, increased G6PDH activity if NADPH levels are low. Choice A can be eliminated because there is a lack of a need for G6PDH activity due to low levels of acyl hydroperoxides and vicine/convicine. Condition C will tend to increase G6PDH activity.
BIOCHEMISTRY PEARLS
![]() Glucose-6-dehydrogenase deficiency is an X-linked inherited disease.
Glucose-6-dehydrogenase deficiency is an X-linked inherited disease.
![]() Glucose-6-dehydrogenase aids in the conversion of glucose-6-phosphate to 6-phosphogluconate with resultant NADPH formation.
Glucose-6-dehydrogenase aids in the conversion of glucose-6-phosphate to 6-phosphogluconate with resultant NADPH formation.
![]() NADPH reducing equivalents act to suppress oxidative damage to the red blood cell membrane. Patients with this deficiency do not have the NADPH reducing ability, leading to red blood cell membrane instability and hemolysis.
NADPH reducing equivalents act to suppress oxidative damage to the red blood cell membrane. Patients with this deficiency do not have the NADPH reducing ability, leading to red blood cell membrane instability and hemolysis.
![]() Consumption of fava beans is only one potential triggering agent of hemolysis in patients with this deficiency. Other etiologies include drugs, chemicals, infection, and DKA.
Consumption of fava beans is only one potential triggering agent of hemolysis in patients with this deficiency. Other etiologies include drugs, chemicals, infection, and DKA.
REFERENCES
McMillan DC, Bolchoz LJC, Jollous, DS. Favism: effect of divicine on rat erythrocyte sulfhydryl status, hexose monophosphate shunt activity, morphology and membrane skeletal proteins. Toxicol Sci. 2001;62:353-359.
Glader B, Schrier S, Mahoney D, Landaw S. Clinical Manifestations of Glucose-6-phosphate Dehydrogenase Deficiency. www.uptodate.com.