Harper’s Illustrated Biochemistry, 29th Edition (2012)
SECTION III. Metabolism of Proteins & Amino Acids
Chapter 27. Biosynthesis of the Nutritionally Nonessential Amino Acids
Victor W. Rodwell, PhD
OBJECTIVES
After studying this chapter, you should be able to:
![]() Explain why the absence from the diet of some amino acids is not deleterious to human health.
Explain why the absence from the diet of some amino acids is not deleterious to human health.
![]() Appreciate the distinction between “essential” and “nutritionally essential” amino acids, and identify the amino acids that are nutritionally nonessential.
Appreciate the distinction between “essential” and “nutritionally essential” amino acids, and identify the amino acids that are nutritionally nonessential.
![]() Name the citric acid cycle and the glycolytic intermediates that are precursors of aspartate, asparagine, glutamate, glutamine, glycine, and serine.
Name the citric acid cycle and the glycolytic intermediates that are precursors of aspartate, asparagine, glutamate, glutamine, glycine, and serine.
![]() Appreciate the key role of transaminases in amino acid metabolism.
Appreciate the key role of transaminases in amino acid metabolism.
![]() Explain the process by which the hydroxyproline and hydroxylysine of proteins are formed.
Explain the process by which the hydroxyproline and hydroxylysine of proteins are formed.
![]() Provide a biochemical explanation for why a severe deprivation of vitamin C (ascorbic acid) results in the nutritional disease scurvy, and describe the clinical consequences of this nutritional disorder.
Provide a biochemical explanation for why a severe deprivation of vitamin C (ascorbic acid) results in the nutritional disease scurvy, and describe the clinical consequences of this nutritional disorder.
![]() Appreciate that, despite the toxicity of selenium, selenocysteine is an essential component of several mammalian proteins.
Appreciate that, despite the toxicity of selenium, selenocysteine is an essential component of several mammalian proteins.
![]() Outline the reaction catalyzed by a mixed-function oxidase.
Outline the reaction catalyzed by a mixed-function oxidase.
![]() Identify the role of tetrahydrobiopterin in tyrosine biosynthesis.
Identify the role of tetrahydrobiopterin in tyrosine biosynthesis.
![]() Indicate the role of a modified tRNA in the cotranslational insertion of selenocysteine into proteins.
Indicate the role of a modified tRNA in the cotranslational insertion of selenocysteine into proteins.
BIOMEDICAL IMPORTANCE
Medical implications of the material in this chapter relate to the amino acid deficiency states that can result if nutritionally essential amino acids are absent from the diet, or are present in inadequate amounts. Amino acid deficiency states endemic in certain regions of West Africa include kwashiorkor, which results when a child is weaned onto a starchy diet poor in protein, and marasmus, in which both caloric intake and specific amino acids are deficient. Patients with short bowel syndrome unable to absorb sufficient quantities of calories and nutrients suffer from significant nutritional and metabolic abnormalities. Both the nutritional disorder scurvy, a dietary deficiency of vitamin C, and specific genetic disorders are associated with an impaired ability of connective tissue to form hydroxyproline and hydroxylysine. The resulting conformational instability of collagen results in bleeding gums, swelling joints, poor wound healing, and ultimately in death. Menkes’ syndrome, characterized by kinky hair and growth retardation, results from a dietary deficiency of copper, which is an essential cofactor for lysyl oxidase, an enzyme that functions in formation of the covalent cross-links that strengthen collagen fibers. Genetic disorders of collagen biosynthesis include several forms of osteogenesis imperfecta, characterized by fragile bones, and Ehlers-Danlos syndrome, a group of connective tissue disorders that result in mobile joints and skin abnormalities due to defects in the genes that encode enzymes including lysyl hydroxylase.
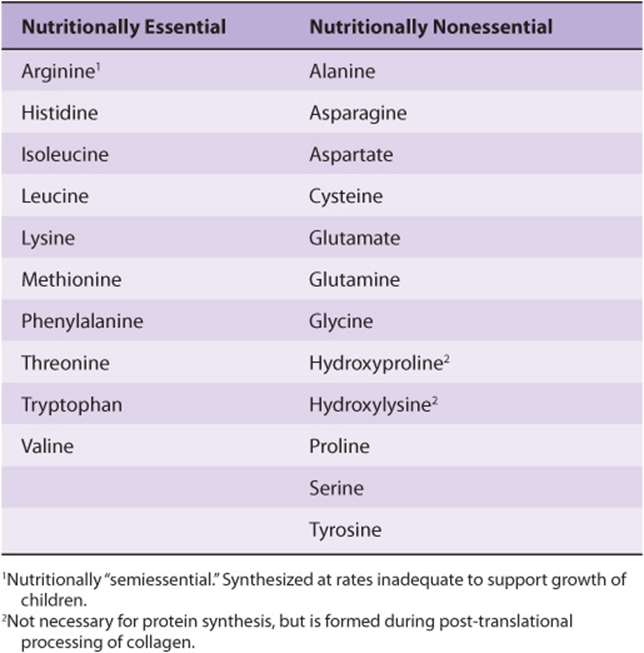
NUTRITIONALLY ESSENTIAL & NUTRITIONALLY NONESSENTIAL AMINO ACIDS
As applied to amino acids, the terms “essential” and “nonessential” are misleading since all 20 common amino acids are essential to ensure health. Of these 20 amino acids, 8 must be present in the human diet, and thus are best termed “nutritionally essential.” The other 12 amino acids are “nutritionally nonessential” since they need not be present in the diet (Table 27-1). The distinction between these two classes of amino acids was established in the 1930s by feeding human subjects purified amino acids in place of protein. Subsequent biochemical investigations revealed the reactions and intermediates involved in the biosynthesis of all 20 amino acids. Amino acid deficiency disorders are endemic in certain regions of West Africa where diets rely heavily on grains that are poor sources of tryptophan and lysine. These nutritional disorders include kwashiorkor, which results when a child is weaned onto a starchy diet poor in protein, and marasmus, in which both caloric intake and specific amino acids are deficient.
TABLE 27–1 Amino Acid Requirements of Humans
Lengthy Metabolic Pathways Form the Nutritionally Essential Amino Acids
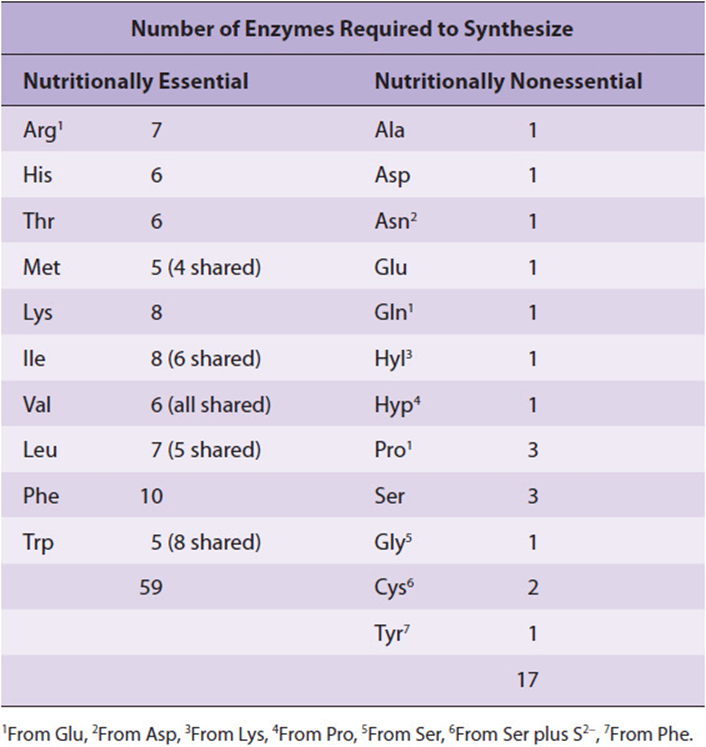
The existence of nutritional requirements suggests that dependence on an external supply of a given nutrient can be of greater survival value than the ability to biosynthesize it. Why? If a specific nutrient is present in the food, an organism that can synthesize it will transfer to its progeny genetic information of negative survival value. The survival value is negative rather than nil because ATP and nutrients are required to synthesize “unnecessary” DNA—even if specific encoded genes are no longer expressed. The number of enzymes required by prokaryotic cells to synthesize the nutritionally essential amino acids is large relative to the number of enzymes required to synthesize the nutritionally nonessential amino acids (Table 27-2). This suggests a survival advantage in retaining the ability to manufacture “easy” amino acids while losing the ability to make “difficult” amino acids. The metabolic pathways that form the nutritionally essential amino acids occur in plants and bacteria, but not in humans, and thus are not discussed. This chapter addresses the reactions and intermediates involved in the biosynthesis by human tissues of the 12 nutritionally nonessential amino acids and selected nutritional and metabolic disorders associated with their metabolism.
TABLE 27–2 Enzymes Required for the Synthesis of Amino Acids from Amphibolic Intermediates
BIOSYNTHESIS OF THE NUTRITIONALLY NONESSENTIAL AMINO ACIDS
Glutamate
The first reaction in biosynthesis of the “glutamate family” of amino acids is the reductive amidation of α-ketoglutarate catalyzed by glutamate dehydrogenase (Figure 27–1). The reaction is shown as unidirectional in the direction of glutamate synthesis because the reaction strongly favors glutamate. This is physiologically important because high concentrations of ammonium ion are cytotoxic.
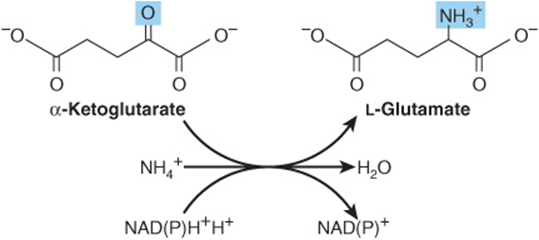
FIGURE 27–1 The glutamate dehydrogenase reaction.
Glutamine
The amidation of glutamate to glutamine catalyzed by glutamine synthetase involves the intermediate formation of γ-glutamyl phosphate (Figure 27–2). Following the ordered binding of glutamate and ATP, glutamate attacks the γ-phosphorus of ATP, forming γ-glutamyl phosphate and ADP. NH4+ then binds, and as NH3 attacks γ-glutamyl phosphate to form a tetrahedral intermediate. Release of Pi and of a proton from the γ-amino group of the tetrahedral intermediate then allows release of the product, glutamine.
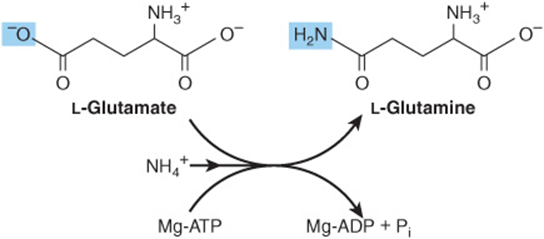
FIGURE 27–2 The glutamine synthetase reaction.
Alanine & Aspartate
Transamination of pyruvate forms alanine (Figure 27–3). Similarly, transamination of oxaloacetate forms aspartate.
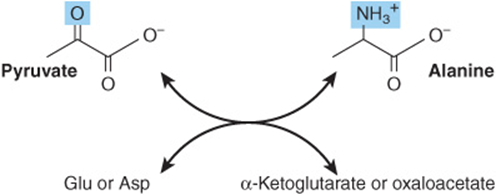
FIGURE 27–3 Formation of alanine by transamination of pyruvate. The amino donor may be glutamate or aspartate. The other product thus is α-ketoglutarate or oxaloacetate.
Glutamate Dehydrogenase, Glutamine Synthetase & Aminotransferases Play Central Roles in Amino Acid Biosynthesis
The combined action of the enzymes glutamate dehydrogenase, glutamine synthetase, and the aminotransferases (Figures 27-1, 27-2 and 27-3) converts inorganic ammonium ion into the α-amino nitrogen of amino acids.
Asparagine
The conversion of aspartate to asparagine, catalyzed by asparagine synthetase (Figure 27–4), resembles the glutamine synthetase reaction (Figure 27–2), but glutamine, rather than ammonium ion, provides the nitrogen. Bacterial asparagine synthetases can, however, also use ammonium ion. The reaction involves the intermediate formation of aspartyl phosphate. The coupled hydrolysis of PPi to Pi by pyrophosphatase ensures that the reaction is strongly favored.
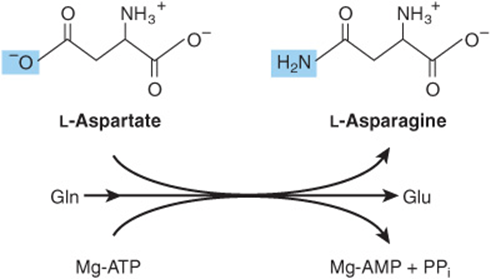
FIGURE 27–4 The asparagine synthetase reaction. Note similarities to and differences from the glutamine synthetase reaction (Figure 27–2).
Serine
Oxidation of the α-hydroxyl group of the glycolytic intermediate 3-phosphoglycerate by 3-phosphoglycerate dehydrogenase converts it to 3-phosphohydroxypyruvate. Transamination and subsequent dephosphorylation then form serine (Figure 27–5).
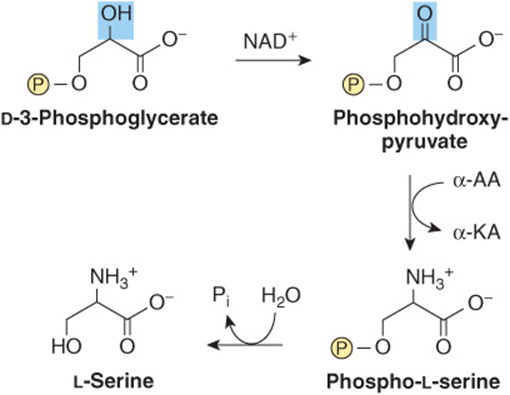
FIGURE 27–5 Serine biosynthesis. (α-AA, α-amino acids; α-KA, α-keto acids.)
Glycine
Glycine aminotransferases can catalyze the synthesis of glycine from glyoxylate and glutamate or alanine. Unlike most aminotransferase reactions, these strongly favor glycine synthesis. Additional important mammalian routes for glycine formation are from choline (Figure 27–6) and from serine (Figure 27–7).
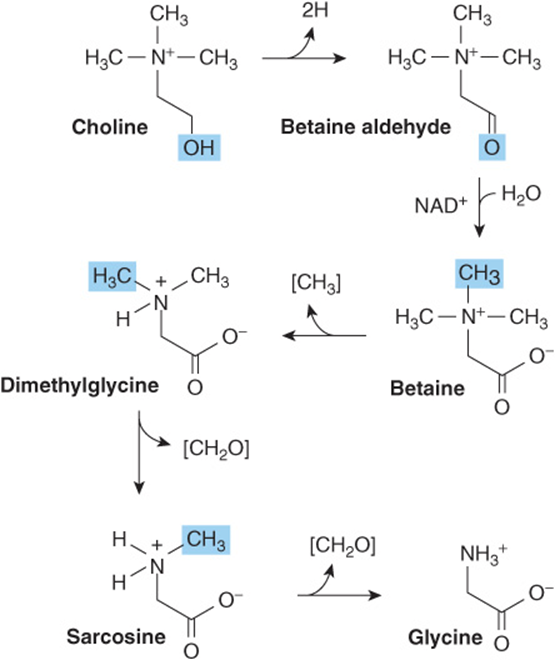
FIGURE 27–6 Formation of glycine from choline. The enzymes that catalyze the reactions shown are choline dehydrogenase, betaine dehydrogenase, betaine-homocysteine N-methyltransferase, sarcosine demethylase, and sarcosine oxidase, respectively.
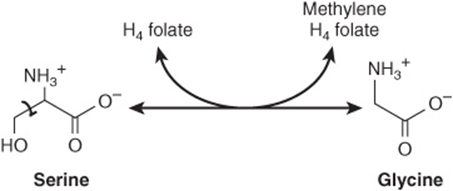
FIGURE 27–7 The serine hydroxymethyltransferase reaction. The reaction is freely reversible. (H4 folate, tetrahydrofolate.)
Proline
The initial reaction of proline biosynthesis converts the γ-carboxyl group of glutamate to the mixed acid anhydride of glutamate γ-phosphate (Figure 27–8). Subsequent reduction forms glutamate γ-semialdehyde, which following spontaneously cyclization is reduced to L-proline.
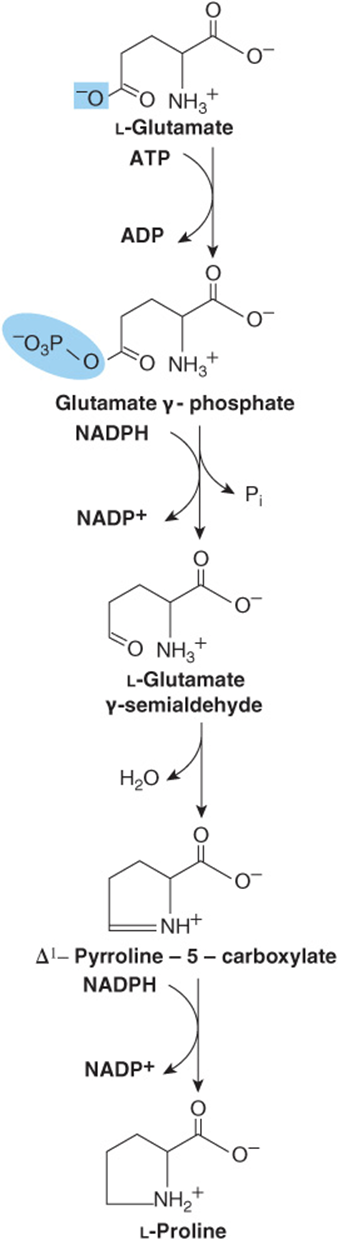
FIGURE 27–8 Biosynthesis of proline from glutamate. The catalysts for these reactions are glutamate 5-kinase, glutamate semialdehyde dehydrogenase, noncatalyzed ring closure, and pyrroline 5-carboxylate reductase.
Cysteine
While not nutritionally essential, cysteine is formed from methionine, which is nutritionally essential. Following conversion of methionine to homocysteine (see Figure 29–19), homocysteine and serine form cystathionine, whose hydrolysis forms cysteine and homoserine (Figure 27–9).
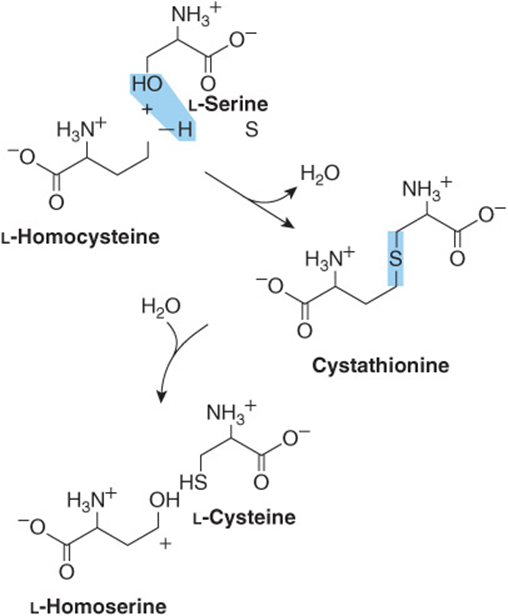
FIGURE 27–9 Conversion of homocysteine and serine to homoserine and cysteine. The sulfur of cysteine derives from methionine and the carbon skeleton from serine.
Tyrosine
Phenylalanine hydroxylase converts phenylalanine to tyrosine (Figure 27–10). If the diet contains adequate quantities of the nutritionally essential amino acid phenylalanine, tyrosine is nutritionally nonessential. However, since the phenylalanine hydroxylase reaction is irreversible, dietary tyrosine cannot replace phenylalanine. Catalysis by this mixed-function oxygenase incorporates one atom of O2 into the para position of phenylalanine and reduces the other atom to water. Reducing power, provided as tetrahydrobiopterin derives ultimately from NADPH (Figure 27–10).
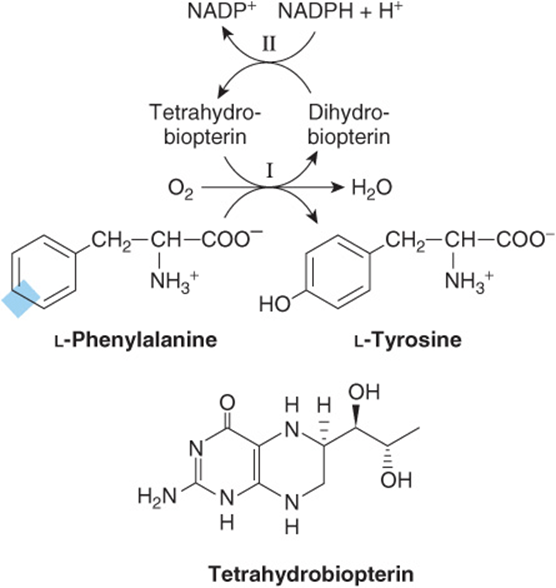
FIGURE 27–10 The phenylalanine hydroxylase reaction. Two distinct enzymatic activities are involved. Activity II catalyzes reduction of dihydrobiopterin by NADPH, and activity I the reduction of O2 to H2O and of phenylalanine to tyrosine. This reaction is associated with several defects of phenylalanine metabolism discussed in Chapter 29.
Hydroxyproline & Hydroxylysine
Hydroxyproline and hydroxylysine occur principally in collagen. Since there is no tRNA for either hydroxylated amino acid, neither dietary hydroxyproline nor dietary hydroxylysine is incorporated during protein synthesis. Peptidyl hydroxyproline and hydroxylysine arise from proline and lysine, but only after these amino acids have been incorporated into peptides. Hydroxylation of peptidyl prolyl and peptidyl lysyl residues, catalyzed by prolyl hydroxylase and lysyl hydroxylase of skin, skeletal muscle, and granulating wounds requires, in addition to the substrate, molecular O2, ascorbate, Fe2+, and α-ketoglutarate (Figure 27–11). For every mole of proline or lysine hydroxylated, one mole of α-ketoglutarate is decarboxylated to succinate. The hydroxylases are mixed-function oxygenases. One atom of O2 is incorporated into proline or lysine, the other into succinate (Figure 27–11). A deficiency of the vitamin C required for these two hydroxylases results in scurvy, in which bleeding gums, swelling joints, and impaired wound healing result from the impaired stability of collagen (see Chapters 5 and 48).
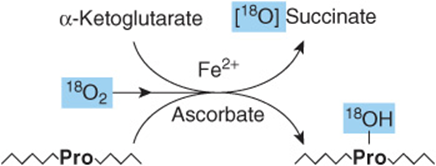
FIGURE 27–11 The prolyl hydroxylase reaction. The substrate is a proline-rich peptide. During the course of the reaction, molecular oxygen is incorporated into both succinate and proline. Lysyl hydroxylase catalyzes an analogous reaction.
Valine, Leucine & Isoleucine
While leucine, valine, and isoleucine are all nutritionally essential amino acids, tissue aminotransferases reversibly interconvert all three amino acids and their corresponding α-keto acids. These α-keto acids thus can replace their amino acids in the diet.
Selenocysteine, the 21st Amino Acid
While the occurrence of selenocysteine (Figure 27–12) in proteins is uncommon, at least 25 human selenoproteins are known. Selenocysteine is present at the active site of several human enzymes that catalyze redox reactions. Examples include thioredoxin reductase, glutathione peroxidase, and the deiodinase that converts thyroxine to triiodothyronine. Where present, selenocysteine participates in the catalytic mechanism of these enzymes. Significantly, the replacement of selenocysteine by cysteine can actually impair catalytic activity. Impairments in human selenoproteins have been implicated in tumorigenesis and atherosclerosis, and are associated with selenium deficiency cardiomyopathy (Keshan disease).
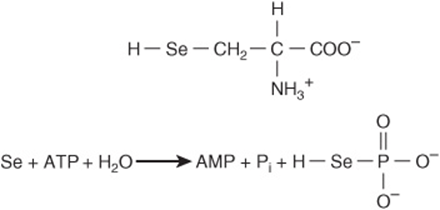
FIGURE 27–12 Selenocysteine (top) and the reaction catalyzed by selenophosphate synthetase (bottom).
Biosynthesis of selenocysteine requires cysteine, selenate (SeO42-), ATP, a specific tRNA, and several enzymes. Serine provides the carbon skeleton of selenocysteine. Selenophosphate, formed from ATP and selenate (Figure 27–12), serves as the selenium donor. Unlike hydroxyproline or hydroxylysine, selenocysteine arises cotranslationally during its incorporation into peptides. The UGA anticodon of the unusual tRNA called tRNASec normally signals STOP. The ability of the protein synthetic apparatus to identify a selenocysteine-specific UGA codon involves the selenocysteine insertion element, a stem-loop structure in the untranslated region of the mRNA. tRNASec is first charged with serine by the ligase that charges tRNASer. Subsequent replacement of the serine oxygen by selenium involves selenophosphate formed by selenophosphate synthetase (Figure 27–12). Successive enzyme-catalyzed reactions convert cysteyl-tRNASec to aminoacrylyl-tRNASec and then to selenocysteyl-tRNAS ec. In the presence of a specific elongation factor that recognizes selenocysteyl-tRNASec, selenocysteine can then be incorporated into proteins.
SUMMARY
![]() All vertebrates can form certain amino acids from amphibolic intermediates or from other dietary amino acids. The intermediates and the amino acids to which they give rise are α-ketoglutarate (Glu, Gln, Pro, Hyp), oxaloacetate (Asp, Asn), and 3-phosphoglycerate (Ser, Gly).
All vertebrates can form certain amino acids from amphibolic intermediates or from other dietary amino acids. The intermediates and the amino acids to which they give rise are α-ketoglutarate (Glu, Gln, Pro, Hyp), oxaloacetate (Asp, Asn), and 3-phosphoglycerate (Ser, Gly).
![]() Cysteine, tyrosine, and hydroxylysine are formed from nutritionally essential amino acids. Serine provides the carbon skeleton and homocysteine the sulfur for cysteine biosynthesis.
Cysteine, tyrosine, and hydroxylysine are formed from nutritionally essential amino acids. Serine provides the carbon skeleton and homocysteine the sulfur for cysteine biosynthesis.
![]() In Scurvy, a nutritional disease that results from a deficiency of vitamin C, impaired hydroxylation of peptidyl proline and peptidyl lysine results in a failure to provide the substrates for cross-linking of maturing collagens.
In Scurvy, a nutritional disease that results from a deficiency of vitamin C, impaired hydroxylation of peptidyl proline and peptidyl lysine results in a failure to provide the substrates for cross-linking of maturing collagens.
![]() Phenylalanine hydroxylase converts phenylalanine to tyrosine. The reaction catalyzed by this mixed function oxidase is irreversible.
Phenylalanine hydroxylase converts phenylalanine to tyrosine. The reaction catalyzed by this mixed function oxidase is irreversible.
![]() Neither dietary hydroxyproline nor hydroxylysine is incorporated into proteins because no codon or tRNA dictates their insertion into peptides.
Neither dietary hydroxyproline nor hydroxylysine is incorporated into proteins because no codon or tRNA dictates their insertion into peptides.
![]() Peptidyl hydroxyproline and hydroxylysine are formed by hydroxylation of peptidyl proline or lysine in reactions catalyzed by mixed-function oxidases that require vitamin C as cofactor.
Peptidyl hydroxyproline and hydroxylysine are formed by hydroxylation of peptidyl proline or lysine in reactions catalyzed by mixed-function oxidases that require vitamin C as cofactor.
![]() Selenocysteine, an essential active site residue in several mammalian enzymes, arises by cotranslational insertion from a previously modified tRNA.
Selenocysteine, an essential active site residue in several mammalian enzymes, arises by cotranslational insertion from a previously modified tRNA.
REFERENCES
Beckett GJ, Arthur JR: Selenium and endocrine systems. J Endocrinol 2005;184:455.
Donovan J, Copeland PR: The efficiency of selenocysteine incorporation is regulated by translation initiation factors. J Mol Biol 2010;400:659.
Kilberg MS: Asparagine synthetase chemotherapy. Annu Rev Biochem 2006;75:629.
Lobanov AV, Hatfield DL, Gladyshev VN: Reduced reliance on the trace element selenium during evolution of mammals. Genome Biol 2008;9:R62.
Scriver CR, Sly WS, Childs B, et al (editors): The Metabolic and Molecular Bases of Inherited Disease, 8th ed. McGraw-Hill, 2001.
Stickel F, Inderbitzin D, Candinas D: Role of nutrition in liver transplantation for end-stage chronic liver disease. Nutr Rev 2008;66:47.