Harper’s Illustrated Biochemistry, 29th Edition (2012)
SECTION VI. Special Topics
Chapter 43. Nutrition, Digestion, & Absorption
David A. Bender, PhD & Peter A. Mayes, PhD, DSc
OBJECTIVES
After studying this chapter, you should be able to:
![]() Describe the digestion and absorption of carbohydrates, lipids, proteins, vitamins, and minerals.
Describe the digestion and absorption of carbohydrates, lipids, proteins, vitamins, and minerals.
![]() Explain how energy requirements can be measured and estimated and how measuring the respiratory quotient permits estimation of the mix of metabolic fuels being oxidized.
Explain how energy requirements can be measured and estimated and how measuring the respiratory quotient permits estimation of the mix of metabolic fuels being oxidized.
![]() Describe the consequences of undernutrition: marasmus, cachexia, and kwashiorkor.
Describe the consequences of undernutrition: marasmus, cachexia, and kwashiorkor.
![]() Explain how protein requirements are determined and why more of some proteins than others is required to maintain nitrogen balance.
Explain how protein requirements are determined and why more of some proteins than others is required to maintain nitrogen balance.
BIOMEDICAL IMPORTANCE
In addition to water, the diet must provide metabolic fuels (mainly carbohydrates and lipids), protein (for growth and turnover of tissue proteins, as well as a source of metabolic fuel), fiber (for bulk in the intestinal lumen), minerals (containing elements with specific metabolic functions), and vitamins and essential fatty acids (organic compounds needed in smaller amounts for other metabolic and physiologic functions). The polysaccharides, triacylglycerols, and proteins that make up the bulk of the diet must be hydrolyzed to their constituent monosaccharides, fatty acids, and amino acids, respectively, before absorption and utilization. Minerals and vitamins must be released from the complex matrix of food before they can be absorbed and utilized.
Globally, undernutrition is widespread, leading to impaired growth, defective immune systems, and reduced work capacity. By contrast, in developed countries, there is excessive food consumption (especially of fat), leading to obesity, and the development of diabetes, cardiovascular disease, and some cancers. Deficiencies of vitamin A, iron, and iodine pose major health concerns in many countries, and deficiencies of other vitamins and minerals are a major cause of ill health. In developed countries nutrient deficiency is rare, although there are vulnerable sections of the population at risk. Intakes of minerals and vitamins that are adequate to prevent deficiency may be inadequate to promote optimum health and longevity.
Excessive secretion of gastric acid, associated with Helicobacter pylori infection, can result in the development of gastric and duodenal ulcers; small changes in the composition of bile can result in crystallization of cholesterol as gallstones; failure of exocrine pancreatic secretion (as in cystic fibrosis) leads to undernutrition and steatorrhea. Lactose intolerance is the result of lactase deficiency, leading to diarrhea and intestinal discomfort when lactose is consumed. Absorption of intact peptides that stimulate antibody responses causes allergic reactions; celiac disease is an allergic reaction to wheat gluten.
DIGESTION & ABSORPTION OF CARBOHYDRATES
The digestion of carbohydrates is by hydrolysis to liberate oligosaccharides, then free mono- and disaccharides. The increase in blood glucose after a test dose of a carbohydrate compared with that after an equivalent amount of glucose (as glucose or from a reference starchy food) is known as the glycemic index. Glucose and galactose have an index of 1 (or 100%), as do lactose, maltose, isomaltose, and trehalose, which give rise to these monosaccharides on hydrolysis. Fructose and the sugar alcohols are absorbed less rapidly and have a lower glycemic index, as does sucrose. The glycemic index of starch varies between near 1 (or 100%) to near 0 as a result of variable rates of hydrolysis, and that of nonstarch polysaccharides is 0. Foods that have a low glycemic index are considered to be more beneficial since they cause less fluctuation in insulin secretion. Resistant starch and nonstarch polysaccharides provide substrates for bacterial fermentation in the large intestine, and the resultant butyrate and other short chain fatty acids provide a significant source of fuel for intestinal enterocytes. There is some evidence that butyrate also has antiproliferative activity, and so provides protection against colorectal cancer.
Amylases Catalyze the Hydrolysis of Starch
The hydrolysis of starch is catalyzed by salivary and pancreatic amylases, which catalyze random hydrolysis of α(1—4) glycoside bonds, yielding dextrins, then a mixture of glucose, maltose, and maltotriose and small branched dextrins (from the branchpoints in amylopectin).
Disaccharidases Are Brush Border Enzymes
The disaccharidases, maltase, sucrase-isomaltase (a bifunctional enzyme catalyzing hydrolysis of sucrose and isomaltose), lactase, and trehalase are located on the brush border of the intestinal mucosal cells, where the resultant monosaccharides and those arising from the diet are absorbed. Congenital deficiency of lactase occurs rarely in infants, leading to lactose intolerance and failure to thrive when fed on breast milk or normal infant formula. Congenital deficiency of sucrase-isomaltase occurs among the Inuit, leading to sucrose intolerance, with persistent diarrhea and failure to thrive when the diet contains sucrose.
In most mammals, and most human beings, lactase activity begins to fall after weaning and is almost completely lost by late adolescence, leading to lactose intolerance. Lactose remains in the intestinal lumen, where it is a substrate for bacterial fermentation to lactate, resulting in abdominal discomfort and diarrhea after consumption of relatively large amounts. In two population groups, people of north European origin and nomadic tribes of sub-Saharan Africa and Arabia, lactase persists after weaning and into adult life. Marine mammals secrete a high-fat milk that contains no carbohydrate, and their pups lack lactase.
There Are Two Separate Mechanisms for the Absorption of Monosaccharides in the Small Intestine
Glucose and galactose are absorbed by a sodium-dependent process. They are carried by the same transport protein (SGLT 1) and compete with each other for intestinal absorption (Figure 43–1). Other monosaccharides are absorbed by carrier-mediated diffusion. Because they are not actively transported, fructose and sugar alcohols are only absorbed down their concentration gradient, and after a moderately high intake, some may remain in the intestinal lumen, acting as a substrate for bacterial fermentation. Large intakes of fructose and sugar alcohols can lead to osmotic diarrhea.
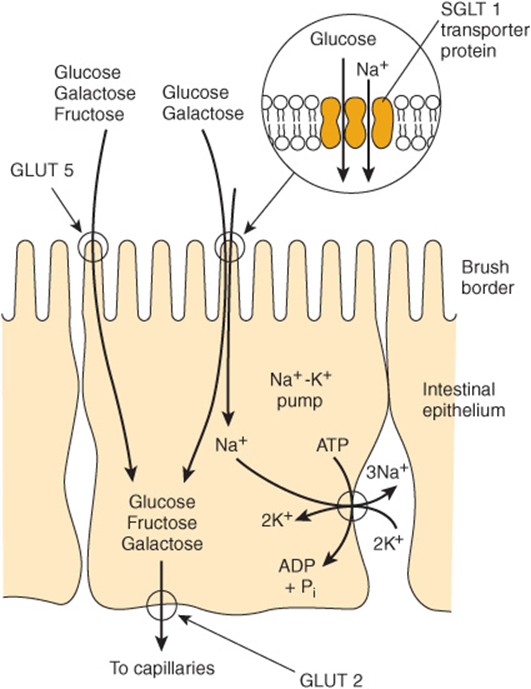
FIGURE 43–1 Transport of glucose, fructose, and galactose across the intestinal epithelium. The SGLT 1 transporter is coupled to the Na+-K+ pump, allowing glucose and galactose to be transported against their concentration gradients. The GLUT 5 Na+-independent facilitative transporter allows fructose, as well as glucose and galactose, to be transported down their concentration gradients. Exit from the cell for all sugars is via the GLUT 2 facilitative transporter.
DIGESTION & ABSORPTION OF LIPIDS
The major lipids in the diet are triacylglycerols and, to a lesser extent, phospholipids. These are hydrophobic molecules and have to be hydrolyzed and emulsified to very small droplets (micelles, 4-6 nm in diameter) before they can be absorbed. The fat-soluble vitamins, A, D, E, and K, and a variety of other lipids (including cholesterol) are absorbed dissolved in the lipid micelles. Absorption of fat-soluble vitamins is impaired on a very low fat diet.
Hydrolysis of triacylglycerols is initiated by lingual and gastric lipases, which attack the sn-3 ester bond forming 1,2-diacylglycerols and free fatty acids, which act as emulsifying agents. Pancreatic lipase is secreted into the small intestine and requires a further pancreatic protein, colipase, for activity. It is specific for the primary ester links—ie, positions 1 and 3 in triacylglycerols—resulting in 2-monoacylglycerols and free fatty acids as the major end products of luminal triacylglycerol digestion. Pancreatic esterase in the intestinal lumen hydrolyzes monoacylglycerols, but they are poor substrates, and only ~25% of ingested triacylglycerol is completely hydrolyzed to glycerol and fatty acids before absorption (Figure 43–2). Bile salts, formed in the liver and secreted in the bile, permit emulsification of the products of lipid digestion into micelles together with dietary phospholipids and cholesterol secreted in the bile (about 2 g/day) as well as dietary cholesterol (about 0.5 g/day). Because the micelles are soluble, they allow the products of digestion, including the fat-soluble vitamins, to be transported through the aqueous environment of the intestinal lumen to come into close contact with the brush border of the mucosal cells, allowing uptake into the epithelium. The bile salts remain in the intestinal lumen, where most are absorbed from the ileum into the enterohepatic circulation (Chapter 26). Within the intestinal epithelium, 1-monoacyglycerols are hydrolyzed to fatty acids and glycerol and 2-monoacylglycerols are reacylated to triacylglycerols via the monoacylglycerol pathway. Glycerol released in the intestinal lumen is absorbed into the hepatic portal vein; glycerol released within the epithelium is reutilized for triacylglycerol synthesis via the normal phosphatidic acid pathway (Chapter 24). Long-chain fatty acids are esterified to yield to triacylglycerol in the mucosal cells and together with the other products of lipid digestion, secreted as chylomicrons into the lymphatics, entering the bloodstream via the thoracic duct (Chapter 25). Short- and medium-chain fatty acids are mainly absorbed into the hepatic portal vein as free fatty acids.
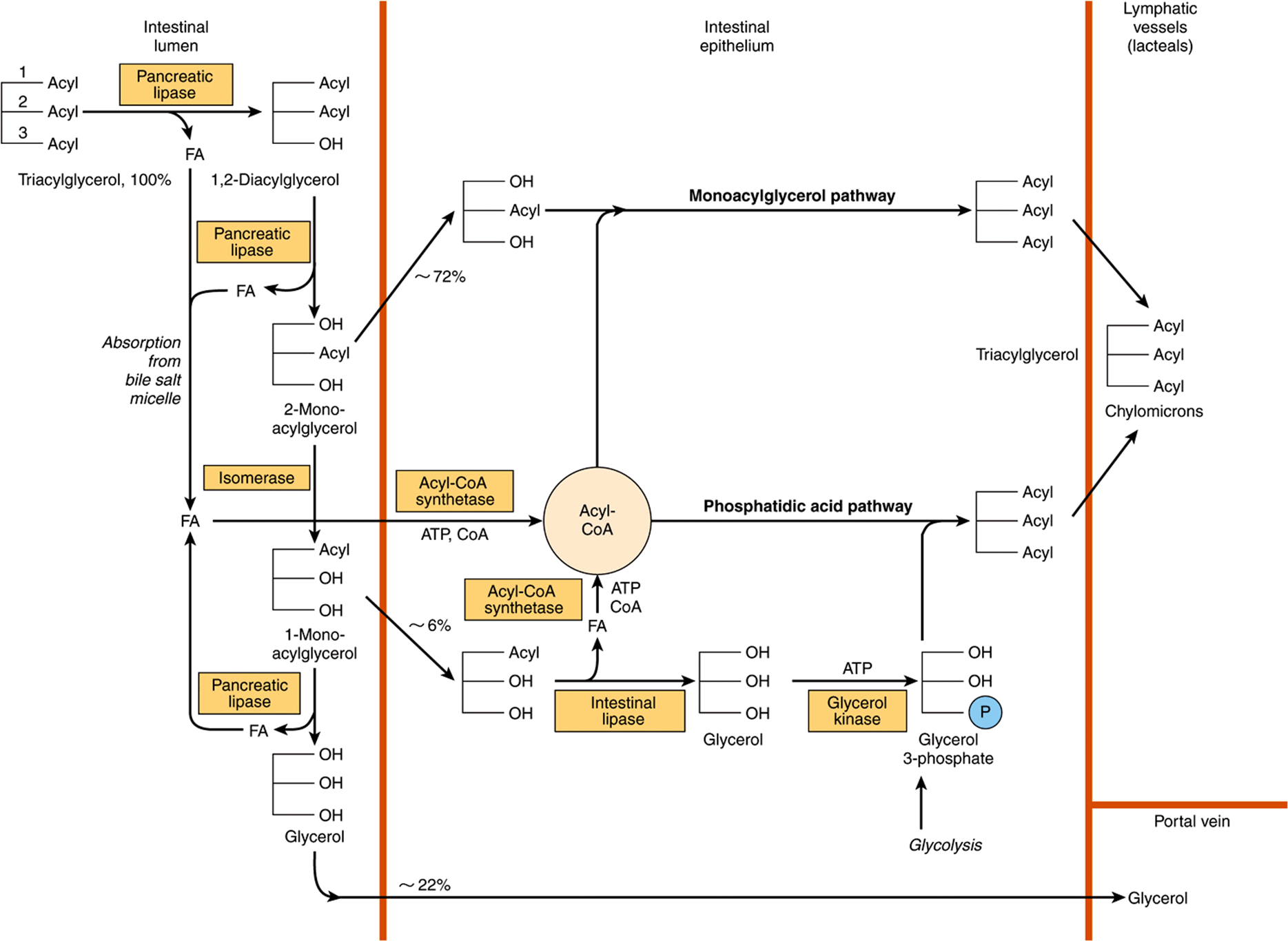
FIGURE 43–2 Digestion and absorption of triacylglycerols. The values given for percentage uptake may vary widely but indicate the relative importance of the three routes shown.
Cholesterol is absorbed dissolved in lipid micelles and is mainly esterified in the intestinal mucosa before being incorporated into chylomicrons. Plant sterols and stanols (in which the B ring is saturated) compete with cholesterol for esterification, but are poor substrates, so that there is an increased amount of unesterified cholesterol in the mucosal cells. Unesterified cholesterol and other sterols are actively transported out of the mucosal cells into the intestinal lumen. This means that plant sterols and stanols effectively inhibit the absorption of not only dietary cholesterol, but also the larger amount that is secreted in the bile, so lowering the whole body cholesterol content, and hence the plasma cholesterol concentration.
DIGESTION & ABSORPTION OF PROTEINS
Native proteins are resistant to digestion because few peptide bonds are accessible to the proteolytic enzymes without prior denaturation of dietary proteins (by heat in cooking and by the action of gastric acid).
Several Groups of Enzymes Catalyze the Digestion of Proteins
There are two main classes of proteolytic digestive enzymes (proteases), with different specificities for the amino acids forming the peptide bond to be hydrolyzed. Endopeptidases hydrolyze peptide bonds between specific amino acids throughout the molecule. They are the first enzymes to act, yielding a larger number of smaller fragments. Pepsin in the gastric juice catalyzes hydrolysis of peptide bonds adjacent to amino acids with bulky side-chains (aromatic and branched-chain amino acids and methionine). Trypsin, chymotrypsin, and elastase are secreted into the small intestine by the pancreas. Trypsin catalyzes hydrolysis of lysine and arginine esters, chymotrypsin esters of aromatic amino acids, and elastase esters of small neutral aliphatic amino acids. Exopeptidases catalyze the hydrolysis of peptide bonds, one at a time, from the ends of peptides. Carboxypeptidases, secreted in the pancreatic juice, release amino acids from the free carboxyl terminal; aminopeptidases, secreted by the intestinal mucosal cells, release amino acids from the amino terminal. Dipeptidases and tripeptidases in the brush border of intestinal mucosal cells catalyze the hydrolysis of di- and tripeptides, which are not substrates for amino- and carboxypeptidases.
The proteases are secreted as inactive zymogens; the active site of the enzyme is masked by a small region of the peptide chain that is removed by hydrolysis of a specific peptide bond. Pepsinogen is activated to pepsin by gastric acid and by activated pepsin. In the small intestine, trypsinogen, the precursor of trypsin, is activated by enteropeptidase, which is secreted by the duodenal epithelial cells; trypsin can then activate chymotrypsinogen to chymotrypsin, proelastase to elastase, procarboxypeptidase to carboxypeptidase, and proaminopeptidase to aminopeptidase.
Free Amino Acids & Small Peptides Are Absorbed by Different Mechanisms
The end product of the action of endopeptidases and exopeptidases is a mixture of free amino acids, di- and tripeptides, and oligopeptides, all of which are absorbed. Free amino acids are absorbed across the intestinal mucosa by sodium-dependent active transport. There are several different amino acid transporters, with specificity for the nature of the amino acid side-chain (large or small, neutral, acidic, or basic). The various amino acids carried by any one transporter compete with each other for absorption and tissue uptake. Dipeptides and tripeptides enter the brush border of the intestinal mucosal cells, where they are hydrolyzed to free amino acids, which are then transported into the hepatic portal vein. Relatively large peptides may be absorbed intact, either by uptake into mucosal epithelial cells (transcellular) or by passing between epithelial cells (paracellular). Many such peptides are large enough to stimulate antibody formation—this is the basis of allergic reactions to foods.
DIGESTION & ABSORPTION OF VITAMINS & MINERALS
Vitamins and minerals are released from food during digestion, although this is not complete, and the availability of vitamins and minerals depends on the type of food and, especially for minerals, the presence of chelating compounds. The fat-soluble vitamins are absorbed in the lipid micelles that are the result of fat digestion; water-soluble vitamins and most mineral salts are absorbed from the small intestine either by active transport or by carrier-mediated diffusion followed by binding to intracellular proteins to achieve concentrative uptake. Vitamin B12 absorption requires a specific transport protein, intrinsic factor (Chapter 44); calcium absorption is dependent on vitamin D; zinc absorption probably requires a zinc-binding ligand secreted by the exocrine pancreas, and the absorption of iron is limited (see below).
Calcium Absorption Is Dependent on Vitamin D
In addition to its role in regulating calcium homeostasis, vitamin D is required for the intestinal absorption of calcium. Synthesis of the intracellular calcium-binding protein, calbindin, required for calcium absorption, is induced by vitamin D. Vitamin D also acts to recruit calcium transporters to the cell surface, so increasing calcium absorption rapidly—a process that is independent of new protein synthesis.
Phytic acid (inositol hexaphosphate) in cereals binds calcium in the intestinal lumen, preventing its absorption. Other minerals, including zinc, are also chelated by phytate. This is mainly a problem among people who consume large amounts of unleavened whole-wheat products; yeast contains an enzyme, phytase, that dephosphorylates phytate, so rendering it inactive. High concentrations of fatty acids in the intestinal lumen, as a result of impaired fat absorption, can also reduce calcium absorption by forming insoluble calcium salts; a high intake of oxalate can sometimes cause deficiency since calcium oxalate is insoluble.
Iron Absorption Is Limited and Strictly Controlled, but Enhanced by Vitamin C and Alcohol
Although iron deficiency is a common problem in both developed and developing countries, about 10% of the population are genetically at risk of iron overload (hemochromatosis), and in order to reduce the risk of adverse effects of nonenzymic generation of free radicals by iron salts, absorption is strictly regulated. Inorganic iron is transported into the mucosal cell by a proton-linked divalent metal ion transporter, and accumulated intracellularly by binding to ferritin. Iron leaves the mucosal cell via a transport protein ferroportin, but only if there is free transferrin in plasma to bind to. Once transferrin is saturated with iron, any that has accumulated in the mucosal cells is lost when the cells are shed. Expression of the ferroportin gene (and possibly also that for the divalent metal ion transporter) is downregulated by hepcidin, a peptide secreted by the liver when body iron reserves are adequate. In response to hypoxia, anemia, or hemorrhage, the synthesis of hepcidin is reduced, leading to increased synthesis of ferroportin and increased iron absorption (Figure 43–3). As a result of this mucosal barrier, only ~10% of dietary iron is absorbed, and only 1-5% from many plant foods (Chapter 50).
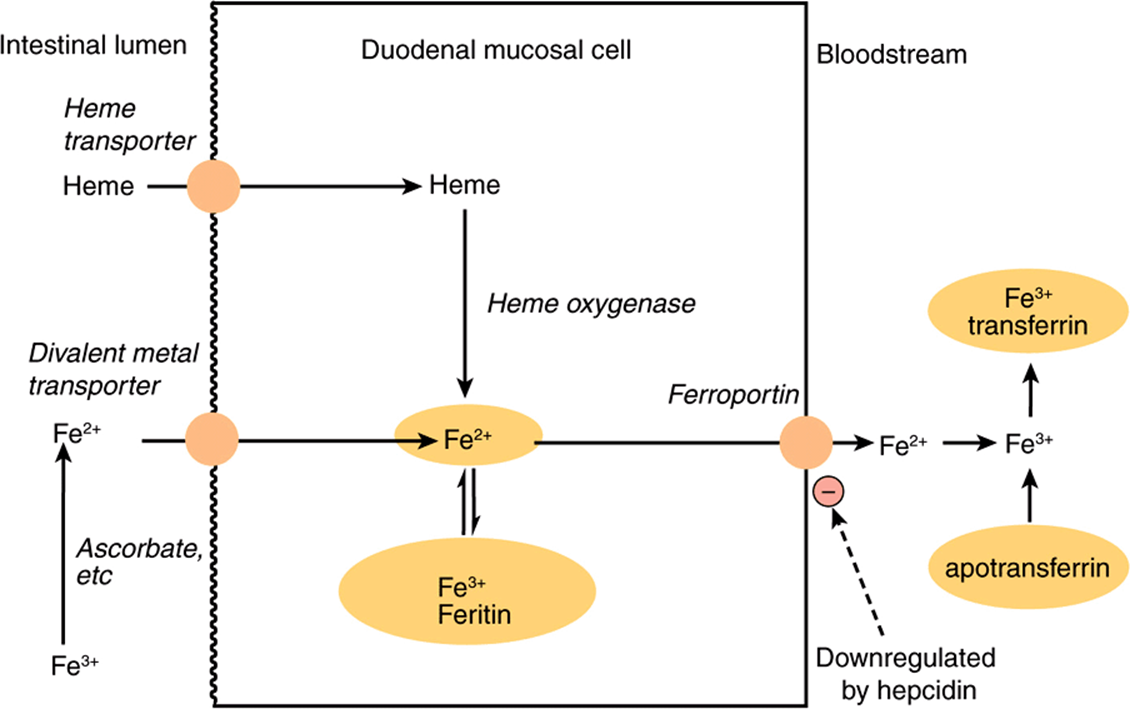
FIGURE 43–3 Absorption of iron. Hepcidin secreted by the liver downregulates synthesis of ferroportin and limits iron absorption.
Inorganic iron is absorbed in the Fe2+ (reduced) state, and hence, the presence of reducing agents enhances absorption. The most effective compound is vitamin C, and while intakes of 40-80 mg of vitamin C/day are more than adequate to meet requirements, an intake of 25-50 mg per meal enhances iron absorption, especially when iron salts are used to treat iron deficiency anemia. Alcohol and fructose also enhance iron absorption. Heme iron from meat is absorbed separately and is considerably more available than inorganic iron. However, the absorption of both inorganic and heme iron is impaired by calcium—a glass of milk with a meal significantly reduces iron availability.
ENERGY BALANCE: OVER- & UNDERNUTRITION
After the provision of water, the body’s first requirement is for metabolic fuels—fats, carbohydrates, and amino acids from proteins (Table 16-1). Food intake in excess of energy expenditure leads to obesity, while intake less than expenditure leads to emaciation and wasting, marasmus, and kwashiorkor. Both obesity and severe undernutrition are associated with increased mortality. The body mass index = weight (in kg)/height2 (in m) is commonly used as a way of expressing relative obesity; a desirable range is between 20 and 25.
Energy Requirements Are Estimated by Measurement of Energy Expenditure
Energy expenditure can be determined directly by measuring heat output from the body, but is normally estimated indirectly from the consumption of oxygen. There is an energy expenditure of ~20 kJ/L of oxygen consumed, regardless of whether the fuel being metabolized is carbohydrate, fat, or protein (Table 16-1).
Measurement of the ratio of the volume of carbon dioxide produced: volume of oxygen consumed (respiratory quotient, RQ) is an indication of the mixture of metabolic fuels being oxidized (Table 16-1).
A more recent technique permits estimation of total energy expenditure over a period of 1-2 weeks, using dual isotopically labeled water, ![]() . 2H is lost from the body only in water, while 18O is lost in both water and carbon dioxide; the difference in the rate of loss of the two labels permits estimation of total carbon dioxide production, and hence oxygen consumption and energy expenditure.
. 2H is lost from the body only in water, while 18O is lost in both water and carbon dioxide; the difference in the rate of loss of the two labels permits estimation of total carbon dioxide production, and hence oxygen consumption and energy expenditure.
Basal metabolic rate (BMR) is the energy expenditure by the body when at rest, but not asleep, under controlled conditions of thermal neutrality, measured about 12 h after the last meal, and depends on weight, age, and gender. Total energy expenditure depends on the BMR, the energy required for physical activity and the energy cost of synthesizing reserves in the fed state. It is therefore possible to estimate an individual’s energy requirement from body weight, age, gender, and level of physical activity. Body weight affects BMR because there is a greater amount of active tissue in a larger body. The decrease in BMR with increasing age, even when body weight remains constant, is the result of muscle tissue replacement by adipose tissue, which is metabolically less active. Similarly, women have a significantly lower BMR than do men of the same body weight and age because women’s bodies contain proportionally more adipose tissue.
Energy Requirements Increase with Activity
The most useful way of expressing the energy cost of physical activities is as a multiple of BMR. Sedentary activities use only about 1.1-1.2 × BMR. By contrast, vigorous exertion, such as climbing stairs, cross-country walking uphill, etc, may use 6-8 × BMR.
Ten Percent of the Energy Yield of a Meal May Be Expended in Forming Reserves
There is a considerable increase in metabolic rate after a meal (diet-induced thermogenesis). A small part of this is the energy cost of secreting digestive enzymes and of active transport of the products of digestion; the major part is the result of synthesizing reserves of glycogen, triacylglycerol, and protein.
There Are Two Extreme Forms of Undernutrition
Marasmus can occur in both adults and children and occurs in vulnerable groups of all populations. Kwashiorkor affects only children and has been reported only in developing countries. The distinguishing feature of kwashiorkor is that there is fluid retention, leading to edema, and fatty infiltration of the liver. Marasmus is a state of extreme emaciation; it is the outcome of prolonged negative energy balance. Not only have the body’s fat reserves been exhausted, but there is wastage of muscle as well, and as the condition progresses there is loss of protein from the heart, liver, and kidneys. The amino acids released by the catabolism of tissue proteins are used as a source of metabolic fuel and as substrates for gluconeogenesis to maintain a supply of glucose for the brain and red blood cells (Chapter 20). As a result of the reduced synthesis of proteins, there is impaired immune response and more risk from infections. Impairment of cell proliferation in the intestinal mucosa occurs, resulting in reduction in the surface area of the intestinal mucosa, and reduction in the absorption of such nutrients as are available.
Patients with Advanced Cancer and AIDS Are Malnourished
Patients with advanced cancer, HIV infection and AIDS, and a number of other chronic diseases are frequently undernourished, a condition called cachexia. Physically, they show all the signs of marasmus, but there is considerably more loss of body protein than that occurs in starvation. The secretion of cytokines in response to infection and cancer increases the catabolism of tissue protein by the ATP-dependent ubiquitin-proteasome pathway, so increasing energy expenditure. This differs from marasmus, in which protein synthesis is reduced, but catabolism in unaffected. Patients are hypermetabolic, ie, they have a considerably increased BMR. In addition to activation of the ubiquitin-proteasome pathway of protein catabolism, three other factors are involved. Many tumors metabolize glucose anaerobically to release lactate. This is then used for gluconeogenesis in the liver, which is energy consuming with a net cost of six ATP for each mol of glucose cycled (see Figure 20–4). There is increased stimulation of mitochondrial uncoupling proteins by cytokines leading to thermogenesis and increased oxidation of metabolic fuels. Futile cycling of lipids occurs because hormone sensitive lipase is activated by a proteoglycan secreted by tumors resulting in liberation of fatty acids from adipose tissue and ATP-expensive reesterification to triacylglycerols in the liver, which are exported in VLDL.
Kwashiorkor Affects Undernourished Children
In addition to the wasting of muscle tissue, loss of intestinal mucosa and impaired immune responses seen in marasmus, children with kwashiorkor show a number of characteristic features. The defining feature is edema, associated with a decreased concentration of plasma proteins. In addition, there is enlargement of the liver as a result of accumulation of fat. It was formerly believed that the cause of kwashiorkor was a lack of protein, with a more or less adequate energy intake; however, analysis of the diets of affected children shows that this is not so. Protein deficiency leads to stunting of growth, and children with kwashiorkor are less stunted than those with marasmus. Furthermore, the edema begins to improve early in treatment, when the child is still receiving a low protein diet.
Very commonly, an infection precipitates kwashiorkor. Superimposed on general food deficiency, there is probably a deficiency of antioxidant nutrients such as zinc, copper, carotene, and vitamins C and E. The respiratory burstin response to infection leads to the production of oxygen and halogen free radicals as part of the cytotoxic action of stimulated macrophages. This added oxidant stress triggers the development of kwashiorkor (see Chapter 54).
PROTEIN & AMINO ACID REQUIREMENTS
Protein Requirements Can Be Determined by Measuring Nitrogen Balance
The state of protein nutrition can be determined by measuring the dietary intake and output of nitrogenous compounds from the body. Although nucleic acids also contain nitrogen, protein is the major dietary source of nitrogen and measurement of total nitrogen intake gives a good estimate of protein intake (mg N × 6.25 = mg protein, as N is 16% of most proteins). The output of N from the body is mainly in urea and smaller quantities of other compounds in urine, undigested protein in feces; significant amounts may also be lost in sweat and shed skin. The difference between intake and output of nitrogenous compounds is known as nitrogen balance. Three states can be defined. In a healthy adult, nitrogen balance is in equilibrium, when intake equals output, and there is no change in the total body content of protein. In a growing child, a pregnant woman, or a person in recovery from protein loss, the excretion of nitrogenous compounds is less than the dietary intake and there is net retention of nitrogen in the body as protein —positive nitrogen balance. In response to trauma or infection, or if the intake of protein is inadequate to meet requirements, there is net loss of protein nitrogen from the body —negative nitrogen balance. Except when replacing protein losses, nitrogen equilibrium can be maintained at any level of protein intake above requirements. A high intake of protein does not lead to positive nitrogen balance; although it increases the rate of protein synthesis, it also increases the rate of protein catabolism, so that nitrogen equilibrium is maintained, albeit with a higher rate of protein turnover. Both protein synthesis and catabolism are ATP expensive, and this increased rate of protein turnover explains the increased diet-induced thermogenesis seen in people consuming a high protein diet.
The continual catabolism of tissue proteins creates the requirement for dietary protein, even in an adult who is not growing; although some of the amino acids released can be reutilized, much is used for gluconeogenesis in the fasting state. Nitrogen balance studies show that the average daily requirement is 0.66 g of protein/kg body weight (0.825 allowing for individual variation), ~55 g/day, or 0.825% of energy intake. Average intakes of protein in developed countries are of the order of 80-100 g/day, ie, 14-15% of energy intake. Because growing children are increasing the protein in the body, they have a proportionally greater requirement than adults and should be in positive nitrogen balance. Even so, the need is relatively small compared with the requirement for protein turnover. In some countries, protein intake is inadequate to meet these requirements, resulting in stunting of growth. There is little or no evidence that athletes and body builders require large amounts of protein; simply consuming more of a normal diet providing about 14% of energy from protein will provide more than enough protein for increased muscle protein synthesis—the main requirement is for an increased energy intake to permit increased protein synthesis.
There Is a Loss of Body Protein in Response to Trauma & Infection
One of the metabolic reactions to a major trauma, such as a burn, a broken limb, or surgery, is an increase in the net catabolism of tissue proteins, both in response to cytokines and glucocorticoid hormones, and as a result of excessive utilization of threonine and cysteine in the synthesis of acute-phase proteins. As much as 6-7% of the total body protein may be lost over 10 days. Prolonged bed rest results in considerable loss of protein because of atrophy of muscles. Protein catabolism may be increased in response to cytokines, and without the stimulus of exercise it is not completely replaced. Lost protein is replaced during convalescence, when there is positive nitrogen balance. Again, as in the case of athletes, a normal diet is adequate to permit this replacement protein synthesis.
The Requirement Is Not Just for Protein, but for Specific Amino Acids
Not all proteins are nutritionally equivalent. More of some is needed to maintain nitrogen balance than others because different proteins contain different amounts of the various amino acids. The body’s requirement is for amino acids in the correct proportions to replace tissue proteins. The amino acids can be divided into two groups: essential and nonessential. There are nine essential or indispensable amino acids, which cannot be synthesized in the body: histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine. If one of these is lacking or inadequate, then regardless of the total intake of protein, it will not be possible to maintain nitrogen balance since there will not be enough of that amino acid for protein synthesis.
Two amino acids, cysteine and tyrosine, can be synthesized in the body, but only from essential amino acid precursors—cysteine from methionine and tyrosine from phenylalanine. The dietary intakes of cysteine and tyrosine thus affect the requirements for methionine and phenylalanine. The remaining 11 amino acids in proteins are considered to be nonessential or dispensable since they can be synthesized as long as there is enough total protein in the diet. If one of these amino acids is omitted from the diet, nitrogen balance can still be maintained. However, only three amino acids, alanine, aspartate, and glutamate, can be considered to be truly dispensable; they are synthesized from common metabolic intermediates (pyruvate, oxaloacetate, and ketoglutarate, respectively). The remaining amino acids are considered as nonessential, but under some circumstances the requirement may outstrip the capacity for their synthesis.
SUMMARY
![]() Digestion involves hydrolyzing food molecules into smaller molecules for absorption through the gastrointestinal epithelium. Polysaccharides are absorbed as monosaccharides, triacylglycerols as 2-monoacylglycerols, fatty acids and glycerol, and proteins as amino acids and small peptides.
Digestion involves hydrolyzing food molecules into smaller molecules for absorption through the gastrointestinal epithelium. Polysaccharides are absorbed as monosaccharides, triacylglycerols as 2-monoacylglycerols, fatty acids and glycerol, and proteins as amino acids and small peptides.
![]() Digestive disorders arise as a result of (1) enzyme deficiency, eg, lactase and sucrase; (2) malabsorption, eg, of glucose and galactose as a result of defects in the Na+-glucose cotransporter (SGLT 1); (3) absorption of unhydrolyzed polypeptides leading to immune responses, eg, as in celiac disease; and (4) precipitation of cholesterol from bile as gallstones.
Digestive disorders arise as a result of (1) enzyme deficiency, eg, lactase and sucrase; (2) malabsorption, eg, of glucose and galactose as a result of defects in the Na+-glucose cotransporter (SGLT 1); (3) absorption of unhydrolyzed polypeptides leading to immune responses, eg, as in celiac disease; and (4) precipitation of cholesterol from bile as gallstones.
![]() In addition to water, the diet must provide metabolic fuels (carbohydrate and fat) for body growth and activity, protein for synthesis of tissue proteins, fiber for bulk in the intestinal contents, minerals for specific metabolic functions (Chapter 44), polyunsaturated fatty acids of the n-3 and n-6 families, and vitamins-organic compounds needed in small amounts for other essential functions (Chapter 44).
In addition to water, the diet must provide metabolic fuels (carbohydrate and fat) for body growth and activity, protein for synthesis of tissue proteins, fiber for bulk in the intestinal contents, minerals for specific metabolic functions (Chapter 44), polyunsaturated fatty acids of the n-3 and n-6 families, and vitamins-organic compounds needed in small amounts for other essential functions (Chapter 44).
![]() Twenty different amino acids are required for protein synthesis, of which nine are essential in the human diet. The quantity of protein required can be determined by studies of nitrogen balance and is affected by protein quality—the amounts of essential amino acids present in dietary proteins compared with the amounts required for tissue porotein synthesis.
Twenty different amino acids are required for protein synthesis, of which nine are essential in the human diet. The quantity of protein required can be determined by studies of nitrogen balance and is affected by protein quality—the amounts of essential amino acids present in dietary proteins compared with the amounts required for tissue porotein synthesis.
![]() Undernutrition occurs in two extreme forms: marasmus, in adults and children, and kwashiorkor in children. Chronic illness can also lead to undernutrition (cachexia) as a result of hypermetabolism.
Undernutrition occurs in two extreme forms: marasmus, in adults and children, and kwashiorkor in children. Chronic illness can also lead to undernutrition (cachexia) as a result of hypermetabolism.
![]() Overnutrition leads to excess energy intake and is associated with chronic noncommunicable diseases such as obesity, type 2 diabetes, atherosclerosis, cancer, and hypertension.
Overnutrition leads to excess energy intake and is associated with chronic noncommunicable diseases such as obesity, type 2 diabetes, atherosclerosis, cancer, and hypertension.
REFERENCES
Bender DA, Bender AE: Nutrition: A Reference Handbook. Oxford University Press, 1997.
Fuller MF, Garllick PJ: Human amino acid requirements: can the controversy be resolved? Ann Rev Nutr 1994;14:217.
Geissler C, Powers HJ (editors): Human Nutrition, 12th ed. Elsevier, 2010.
Gibney MJ, Lanham-New S, Cassidy A, et al: Introduction to Human Nutrition, The Nutrition Society Textbook Series, 2nd ed. Wiley-Blackwell, 2009.
Institute of Medicine: Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). National Academies Press, 2002.
Pencharz PB, Ball RO: Different approaches to define individual amino acid requirements. Ann Rev Nutr 2003;23:101.
Royal College of Physicians: Nutrition and Patients—A Doctor’s Responsibility. Royal College of Physicians, 2002.
Swallow DM: Genetic influences on carbohydrate digestion. Nutr Res Rev 2003;16:37.
World Health Organization Technical Report Series 894: Obesity—Preventing and Managing the Global Epidemic. WHO, 2000.
World Health Organization Technical Report Series 916: Diet and the Prevention of Chronic Diseases. WHO, 2003.
World Health Organization Technical report Series 935: Protein and Amino Acid Requirements in Human Nutrition. WHO, 2007.