CHEMISTRY THE CENTRAL SCIENCE
2 ATOMS, MOLECULES, AND IONS
2.2 THE DISCOVERY OF ATOMIC STRUCTURE
Dalton based his conclusions about atoms on chemical observations made in the laboratory. Neither he nor those who followed him during the century after his work was published had any direct evidence for the existence of atoms. Today however, we can measure the properties of individual atoms and even provide images of them (![]() FIGURE 2.2).
FIGURE 2.2).
As scientists developed methods for probing the nature of matter, the supposedly indivisible atom began to show signs of a more complex structure, and today we know that the atom is composed of subatomic particles. Before we summarize the current model, we briefly consider a few of the landmark discoveries that led to that model. We will see that the atom is composed in part of electrically charged particles, some with a positive charge and some with a negative charge. As we discuss the development of our current model of the atom, keep in mind this fact: Particles with the same charge repel one another, whereas particles with unlike charges attract one another.
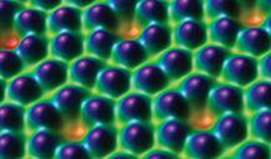
![]() FIGURE 2.2 An image of the surface of silicon. The image was obtained by a technique called scanning tunneling microscopy. The color was added to the image by computer to help distinguish its features. Each purple sphere is a silicon atom.
FIGURE 2.2 An image of the surface of silicon. The image was obtained by a technique called scanning tunneling microscopy. The color was added to the image by computer to help distinguish its features. Each purple sphere is a silicon atom.
Cathode Rays and Electrons
During the mid-1800s, scientists began to study electrical discharge through a glass tube pumped almost empty of air (![]() FIGURE 2.3). When a high voltage was applied to the electrodes in the tube, radiation was produced between the electrodes. This radiation, called cathode rays, originated at the negative electrode and traveled to the positive electrode. Although the rays could not be seen, their presence was detected because they cause certain materials to fluoresce, or to give off light.
FIGURE 2.3). When a high voltage was applied to the electrodes in the tube, radiation was produced between the electrodes. This radiation, called cathode rays, originated at the negative electrode and traveled to the positive electrode. Although the rays could not be seen, their presence was detected because they cause certain materials to fluoresce, or to give off light.
![]() GO FIGURE
GO FIGURE
How do we know that the cathode rays travel from cathode to anode?
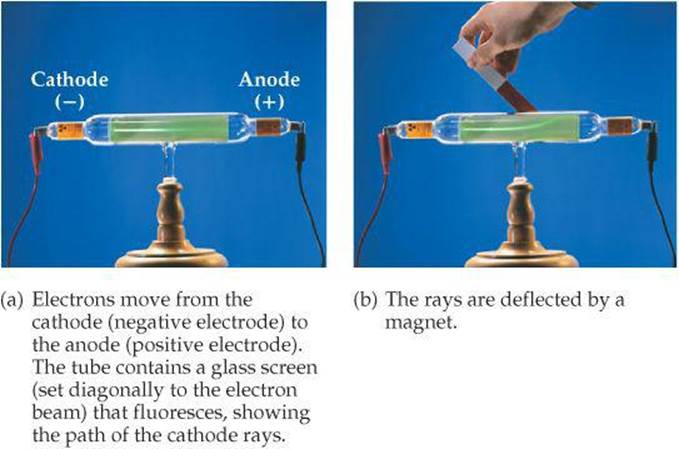
![]() Figure 2.3 Cathode-ray tube.
Figure 2.3 Cathode-ray tube.
![]() GO FIGURE
GO FIGURE
If no magnetic field were applied, would you expect the electron beam to be deflected upward or downward by the electric field?
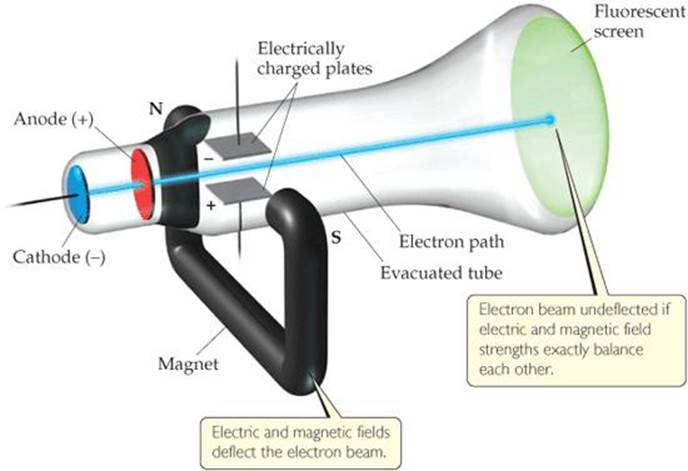
![]() Figure 2.4 Cathode-ray tube with perpendicular magnetic and electric fields. The cathode rays (electrons) originate at the cathode and are accelerated toward the anode, which has a hole in its center. A narrow beam of electrons passes through the hole and travels to the fluorescent screen. The strengths of the electric and magnetic fields are adjusted so their effects cancel each other allowing the beam to travel a straight path.
Figure 2.4 Cathode-ray tube with perpendicular magnetic and electric fields. The cathode rays (electrons) originate at the cathode and are accelerated toward the anode, which has a hole in its center. A narrow beam of electrons passes through the hole and travels to the fluorescent screen. The strengths of the electric and magnetic fields are adjusted so their effects cancel each other allowing the beam to travel a straight path.
Experiments showed that cathode rays are deflected by electric or magnetic fields in a way consistent with their being a stream of negative electrical charge. The British scientist J. J. Thomson (1856–1940) observed that cathode rays are the same regardless of the identity of the cathode material. In a paper published in 1897, Thomson described cathode rays as streams of negatively charged particles. His paper is generally accepted as the “discovery” of what became known as the electron.
Thomson constructed a cathode-ray tube having a hole in the anode through which a beam of electrons passed. Electrically charged plates and a magnet were positioned perpendicular to the electron beam, and a fluorescent screen was located at one end (![]() FIGURE 2.4). The elecetric field deflected the rays in one direction, and the mag netic field deflected them in the opposite direction. Thomson adjusted the strengths of the fields so that the effects balanced each other, allowing the electrons to travel in a straight path to the screen. Knowing the strengths that resulted in the straight path made it possible to calculate a value of 1.76 × 108 coulombs per gram for the ratio of the electron's electrical charge to its mass.*
FIGURE 2.4). The elecetric field deflected the rays in one direction, and the mag netic field deflected them in the opposite direction. Thomson adjusted the strengths of the fields so that the effects balanced each other, allowing the electrons to travel in a straight path to the screen. Knowing the strengths that resulted in the straight path made it possible to calculate a value of 1.76 × 108 coulombs per gram for the ratio of the electron's electrical charge to its mass.*
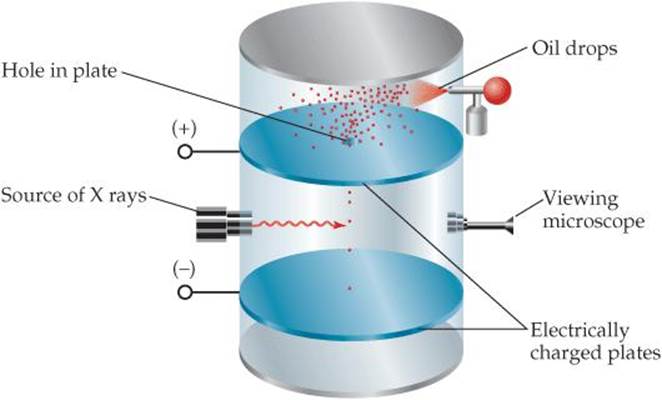
![]() Figure 2.5 Millikan's oil-drop experiment used to measure the charge of the electron. Small drops of oil were allowed to fall between electrically charged plates. The drops picked up extra electrons as a result of irradiation by X-rays and so became negatively charged. Millikan measured how varying the voltage between the plates affected the rate of fall. From these data he calculated the negative charge on the drops. Because the charge on any drop was always some integral multiple of 1.602 × 10–19 C, Millikan deduced this value to be the charge of a single electron.
Figure 2.5 Millikan's oil-drop experiment used to measure the charge of the electron. Small drops of oil were allowed to fall between electrically charged plates. The drops picked up extra electrons as a result of irradiation by X-rays and so became negatively charged. Millikan measured how varying the voltage between the plates affected the rate of fall. From these data he calculated the negative charge on the drops. Because the charge on any drop was always some integral multiple of 1.602 × 10–19 C, Millikan deduced this value to be the charge of a single electron.
Once the charge-to-mass ratio of the electron was known, measuring either quantity allowed scientists to calculate the other. In 1909, Robert Millikan (1868–1953) of the University of Chicago succeeded in measuring the charge of an electron by performing the experiment described in ![]() FIGURE 2.5. He then calculated the mass of the electron by using his experimental value for the charge, 1.602 × 10–19 C, and Thomson's charge-to-mass ratio, 1.76 × 108 C/g:
FIGURE 2.5. He then calculated the mass of the electron by using his experimental value for the charge, 1.602 × 10–19 C, and Thomson's charge-to-mass ratio, 1.76 × 108 C/g:
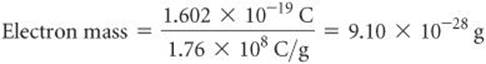
This result agrees well with the currently accepted value for the electron mass, 9.10938 × 10–28 g. This mass is about 2000 times smaller than that of hydrogen, the lightest atom.
Radioactivity
In 1896 the French scientist Henri Becquerel (1852–1908) discovered that a compound of uranium spontaneously emits high-energy radiation. This spontaneous emission of radiation is called radioactivity. At Becquerel's suggestion, Marie Curie (![]() FIGURE 2.6) and her husband, Pierre, began experiments to isolate the radioactive components of the compound.
FIGURE 2.6) and her husband, Pierre, began experiments to isolate the radioactive components of the compound.
Further study of radioactivity, principally by the British scientist Ernest Rutherford (![]() FIGURE 2.7), revealed three types of radiation: alpha (α), beta (β), and gamma (γ). The paths of α and β radiation are bent by an electric field, although in opposite directions; γ radiation is unaffected by the field (
FIGURE 2.7), revealed three types of radiation: alpha (α), beta (β), and gamma (γ). The paths of α and β radiation are bent by an electric field, although in opposite directions; γ radiation is unaffected by the field (![]() FIGURE 2.8).
FIGURE 2.8).
Rutherford showed that α and β rays consist of fast-moving particles. In fact, β particles are high-speed electrons and can be considered the radioactive equivalent of cathode rays. They are attracted to a positively charged plate. The α particles have a positive charge and are attracted to a negative plate. In units of the charge of the electron, β particles have a charge of 1- and α particles a charge of 2 +. Each α particle has a mass about 7400 times that of an electron. Gamma radiation is high-energy radiation similar to X-rays; it does not consist of particles and carries no charge.

![]() Figure 2.6 Marie Sklodowska Curie (1867–1934). When Marie Curie presented her doctoral thesis, it was described as the greatest single contribution of any doctoral thesis in the history of science. In 1903 Henri Becquerel, Maire Curie, and her husband, Pierre, were jointly awarded the Nobel Prize in Physics for their pioneering work on radioactivity (a term she introduced). In 1911 Marie Curie won a second Nobel Prize, this time in chemistry for her discovery of the elements polonium and radium.
Figure 2.6 Marie Sklodowska Curie (1867–1934). When Marie Curie presented her doctoral thesis, it was described as the greatest single contribution of any doctoral thesis in the history of science. In 1903 Henri Becquerel, Maire Curie, and her husband, Pierre, were jointly awarded the Nobel Prize in Physics for their pioneering work on radioactivity (a term she introduced). In 1911 Marie Curie won a second Nobel Prize, this time in chemistry for her discovery of the elements polonium and radium.

![]() Figure 2.7 Ernest Rutherford (1871–1937). In 1895, Rutherford was awarded a position at Cambridge University in England, where he worked with J. J. Thomson. In 1898 he moved to McGill University in Montreal, where he did the research on radioactivity that led to his 1908 Nobel Prize in Chemistry. In 1907 Rutherford returned to England as a faculty member at Manchester University, where in 1910 he performed his famous α-particle scattering experiments. In 1992 his native New Zealand honored him by putting his likeness on their $100 currency note.
Figure 2.7 Ernest Rutherford (1871–1937). In 1895, Rutherford was awarded a position at Cambridge University in England, where he worked with J. J. Thomson. In 1898 he moved to McGill University in Montreal, where he did the research on radioactivity that led to his 1908 Nobel Prize in Chemistry. In 1907 Rutherford returned to England as a faculty member at Manchester University, where in 1910 he performed his famous α-particle scattering experiments. In 1992 his native New Zealand honored him by putting his likeness on their $100 currency note.
The Nuclear Model of the Atom
With growing evidence that the atom is composed of smaller particles, attention was given to how the particles fit together. During the early 1900s, Thomson reasoned that because electrons contribute only a very small fraction of an atom's mass they probably were responsible for an equally small fraction of the atom's size. He proposed that the atom consisted of a uniform positive sphere of matter in which the electrons were embedded like raisins in a pudding or seeds in a watermelon (![]() FIGURE 2.9). This plum-pudding model, named after a traditional English dessert, was very short-lived.
FIGURE 2.9). This plum-pudding model, named after a traditional English dessert, was very short-lived.
In 1910, Rutherford was studying the angles at which α particles were deflected, or scattered, as they passed through a thin sheet of gold foil (![]() FIGURE 2.10). He discovered that almost all the particles passed directly through the foil without deflection, with a few particles deflected about 1 degree, consistent with Thomson's plum-pudding model. For the sake of completeness, Rutherford suggested that Ernest Marsden, an undergraduate student working in the laboratory, look for scattering at large angles. To everyone's surprise, a small amount of scattering was observed at large angles, with some particles scattered back in the direction from which they had come. The explanation for these results was not immediately obvious, but they were clearly inconsistent with Thomson's plum-pudding model.
FIGURE 2.10). He discovered that almost all the particles passed directly through the foil without deflection, with a few particles deflected about 1 degree, consistent with Thomson's plum-pudding model. For the sake of completeness, Rutherford suggested that Ernest Marsden, an undergraduate student working in the laboratory, look for scattering at large angles. To everyone's surprise, a small amount of scattering was observed at large angles, with some particles scattered back in the direction from which they had come. The explanation for these results was not immediately obvious, but they were clearly inconsistent with Thomson's plum-pudding model.
![]() GO FIGURE
GO FIGURE
Which of the three kinds of radiation shown consists of electrons? Why are these rays deflected to a greater extent than the others?
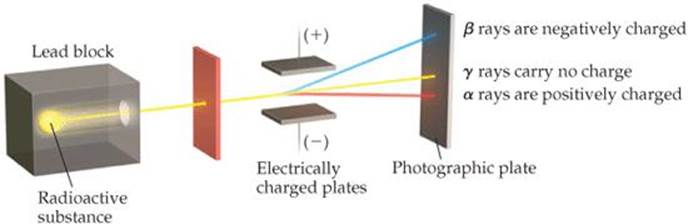
![]() Figure 2.8 Behavior of alpha (α), beta (β), and gamma (γ) rays in an electric field.
Figure 2.8 Behavior of alpha (α), beta (β), and gamma (γ) rays in an electric field.
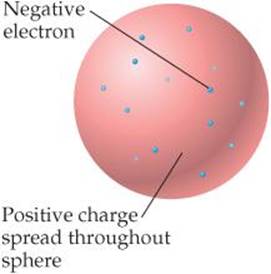
![]() Figure 2.9 J. J. Thomson's plum-pudding model of the atom. Ernest Rutherford proved this model wrong.
Figure 2.9 J. J. Thomson's plum-pudding model of the atom. Ernest Rutherford proved this model wrong.
![]() GO FIGURE
GO FIGURE
What is the charge on the particles that form the beam?
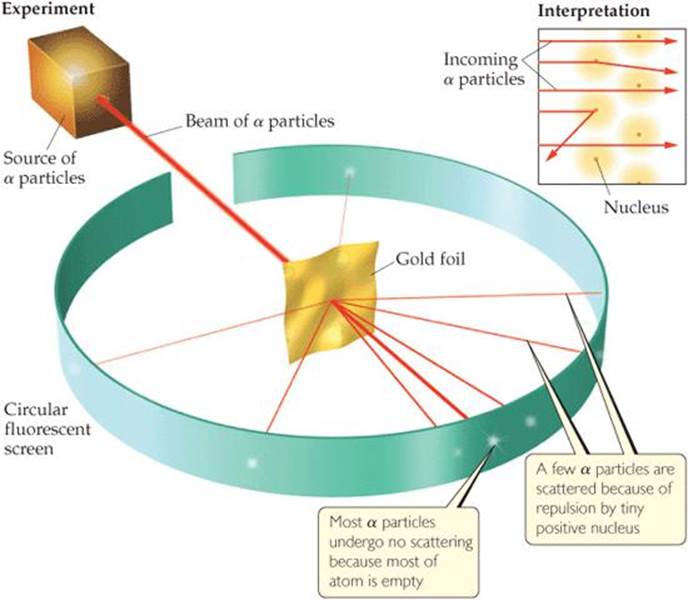
![]() Figure 2.10 Rutherford's α-scattering experiment. When α particles pass through a gold foil, most pass through undeflected but some are scattered, a few at very large angles. According to the plum-pudding model of the atom, the particles should experience only very minor deflections. The nuclear model of the atom explains why a few α particles are deflected at large angles. For clarity, the nuclear atom is shown here as a colored sphere, but most of the space around the nucleus is empty except for the tiny electrons moving around.
Figure 2.10 Rutherford's α-scattering experiment. When α particles pass through a gold foil, most pass through undeflected but some are scattered, a few at very large angles. According to the plum-pudding model of the atom, the particles should experience only very minor deflections. The nuclear model of the atom explains why a few α particles are deflected at large angles. For clarity, the nuclear atom is shown here as a colored sphere, but most of the space around the nucleus is empty except for the tiny electrons moving around.
Rutherford explained the results by postulating the nuclear model of the atom, a model in which most of the mass of each gold atom and all of its positive charge reside in a very small, extremely dense region that he called the nucleus. He postulated further that most of the volume of an atom is empty space in which electrons move around the nucleus. In the α-scattering experiment, most of the particles passed through the foil unscattered because they did not encounter the minute nucleus of any gold atom. Occasionally, however, an α particle came close to a gold nucleus. The repulsion between the highly positive charge of the gold nucleus and the positive charge of the α particle was then strong enough to deflect the particle, as shown in Figure 2.10.
Subsequent experiments led to the discovery of positive particles (protons) and neutral particles (neutrons) in the nucleus. Protons were discovered in 1919 by Rutherford and neutrons in 1932 by British scientist James Chadwick (1891–1972). Thus, the atom is composed of electrons, protons, and neutrons.
![]() GIVE IT SOME THOUGHT
GIVE IT SOME THOUGHT
What happens to most of the α particles that strike the gold foil in Rutherford's experiment? Why do they behave that way?