CHEMISTRY THE CENTRAL SCIENCE
13 PROPERTIES OF SOLUTIONS
13.2 SATURATED SOLUTIONS AND SOLUBILITY
As a solid solute begins to dissolve in a solvent, the concentration of solute particles in solution increases, increasing the chances that some solute particles will collide with the surface of the solid and reattach. This process, which is the opposite of the solution process, is calledcrystallization. Thus, two opposing processes occur in a solution in contact with undissolved solute. This situation is represented in a chemical equation by two half arrows:

![]() GO FIGURE
GO FIGURE
What two processes are represented in this figure, and what are their relative rates at equilibrium?
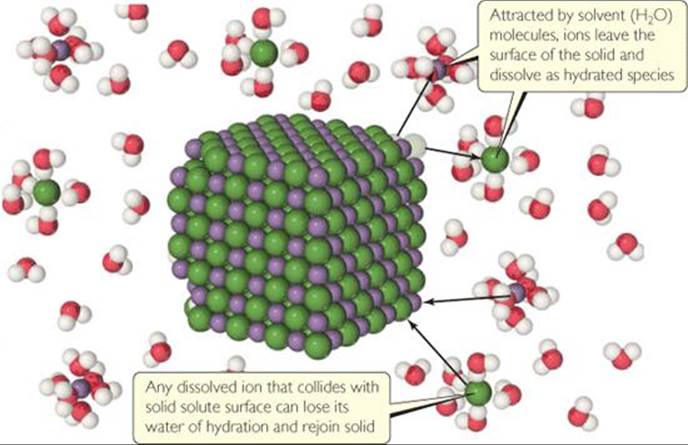
![]() FIGURE 13.8 Dynamic equilibrium in a saturated solution with excess ionic solute.
FIGURE 13.8 Dynamic equilibrium in a saturated solution with excess ionic solute.
When the rates of these opposing processes become equal, a dynamic equilibrium is established and there is no further increase in the amount of solute in solution. (![]() FIGURE 13.8)
FIGURE 13.8)
A solution that is in equilibrium with undissolved solute is saturated. Additional solute will not dissolve if added to a saturated solution. The amount of solute needed to form a saturated solution in a given quantity of solvent is known as the solubility of that solute. That is, the solubility of a given solute in a given solvent is the maximum amount of the solute that can dissolve in a given amount of the solvent at a specified temperature, given that excess solute is present. For example, the solubility of NaCl in water at 0 °C is 35.7 g per 100 mL of water. This is the maximum amount of NaCl that can be dissolved in water to give a stable equilibrium solution at that temperature.
If we dissolve less solute than the amount needed to form a saturated solution, the solution is unsaturated. Thus, a solution containing 10.0 g of NaCl per 100 mL of water at 0 °C is unsaturated because it has the capacity to dissolve more solute.
Under suitable conditions it is possible to form solutions that contain a greater amount of solute than needed to form a saturated solution. Such solutions are supersaturated. For example, when a saturated solution of sodium acetate is made at a high temperature and then slowly cooled, all of the solute may remain dissolved even though its solubility decreases as the temperature decreases. Because the solute in a supersaturated solution is present in a concentration higher than the equilibrium concentration, supersaturated solutions are unstable. For crystallization to occur, however, the solute particles must arrange themselves properly to form crystals. The addition of a small crystal of the solute (a seed crystal) provides a template for crystallization of the excess solute, leading to a saturated solution in contact with excess solid (![]() FIGURE 13.9).
FIGURE 13.9).
![]() GIVE IT SOME THOUGHT
GIVE IT SOME THOUGHT
What happens if solute is added to a saturated solution?
![]() GO FIGURE
GO FIGURE
What is the evidence that the solution in the left photograph is supersaturated?
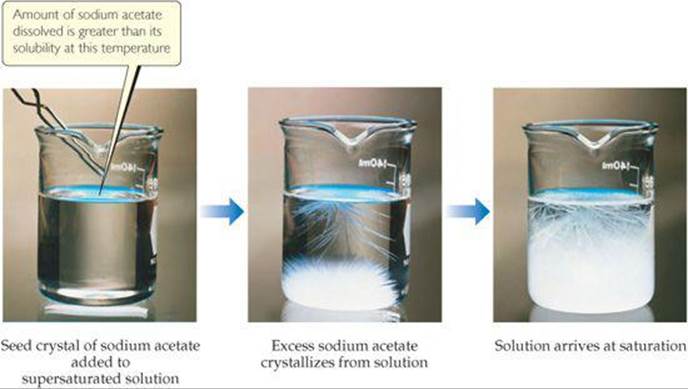
![]() FIGURE 13.9 Precipitation from a supersaturated sodium acetate solution. The solution on the left was formed by dissolving about 170 g of the salt in 100 mL of water at 100 °C and then slowly cooling it to 20 °C. Because the solubility of sodium acetate in water at 20 °C is 46 g per 100 mL of water, the solution is supersaturated. Addition of a sodium acetate crystal causes the excess solute to crystallize from solution.
FIGURE 13.9 Precipitation from a supersaturated sodium acetate solution. The solution on the left was formed by dissolving about 170 g of the salt in 100 mL of water at 100 °C and then slowly cooling it to 20 °C. Because the solubility of sodium acetate in water at 20 °C is 46 g per 100 mL of water, the solution is supersaturated. Addition of a sodium acetate crystal causes the excess solute to crystallize from solution.