CHEMISTRY THE CENTRAL SCIENCE
16 ACID–BASE EQUILIBRIA
16.11 LEWIS ACIDS AND BASES
For a substance to be a proton acceptor (a Brønsted–Lowry base), it must have an unshared pair of electrons for binding the proton, as, for example, in NH3. Using Lewis structures, we can write the reaction between H+ and NH3 as
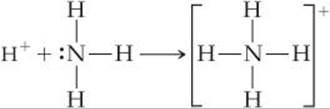
G. N. Lewis was the first to notice this aspect of acid–base reactions. He proposed a definition of acid and base that emphasizes the shared electron pair: A Lewis acid is an electron-pair acceptor, and a Lewis base is an electron-pair donor.
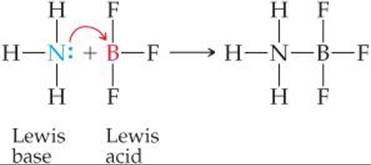
Every base that we have discussed thus far—whether OH–, H2O, an amine, or an anion—is an electron-pair donor. Everything that is a base in the Brønsted–Lowry sense (a proton acceptor) is also a base in the Lewis sense (an electron-pair donor). In the Lewis theory, however, a base can donate its electron pair to something other than H+. The Lewis definition therefore greatly increases the number of species that can be considered acids; in other words, H+ is a Lewis acid but not the only one. For example, the reaction between NH3 and BF3 occurs because BF3 has a vacant orbital in its valence shell. ![]() (Section 8.7) It therefore acts as an electron-pair acceptor (a Lewis acid) toward NH3, which donates the electron pair:
(Section 8.7) It therefore acts as an electron-pair acceptor (a Lewis acid) toward NH3, which donates the electron pair:
![]() GIVE IT SOME THOUGHT
GIVE IT SOME THOUGHT
What feature must any molecule or ion have in order to act as a Lewis base?
Our emphasis throughout this chapter has been on water as the solvent and on the proton as the source of acidic properties. In such cases we find the Brønsted–Lowry definition of acids and bases to be the most useful. In fact, when we speak of a substance as being acidic or basic, we are usually thinking of aqueous solutions and using these terms in the Arrhenius or Brønsted–Lowry sense. The advantage of the Lewis definitions of acid and base is that they allow us to treat a wider variety of reactions, including those that do not involve proton transfer, as acid–base reactions. To avoid confusion, a substance such as BF3 is rarely called an acid unless it is clear from the context that we are using the term in the sense of the Lewis definition. Instead, substances that function as electron-pair acceptors are referred to explicitly as “Lewis acids.”
Lewis acids include molecules that, like BF3, have an incomplete octet of electrons. In addition, many simple cations can function as Lewis acids. For example, Fe3+ interacts strongly with cyanide ions to form the ferricyanide ion:
![]()
The Fe3+ ion has vacant orbitals that accept the electron pairs donated by the cyanide ions. (We will learn more in Chapter 23 about just which orbitals are used by the Fe3+ ion.) The metal ion is highly charged, too, which contributes to the interaction with CN– ions.
Some compounds containing multiple bonds can behave as Lewis acids. For example, the reaction of carbon dioxide with water to form carbonic acid (H2CO3) can be pictured as an attack by a water molecule on CO2, in which the water acts as an electron-pair donor and the CO2 as an electron-pair acceptor:
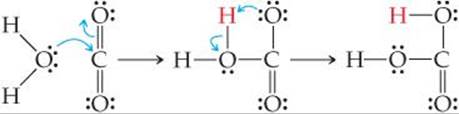
One electron pair of one of the carbon-oxygen double bonds is moved onto the oxygen, leaving a vacant orbital on the carbon, which means the carbon can accept an electron pair donated by H2O. The initial acid–base product rearranges by transferring a proton from the water oxygen to a carbon dioxide oxygen, forming carbonic acid.
The hydrated cations we encountered in Section 16.9, such as [Fe(H2O)6]3+ in Figure 16.16, form through the reaction between the cation acting as a Lewis acid and the water molecules acting as Lewis bases. When a water molecule interacts with the positively charged metal ion, electron density is drawn from the oxygen (![]() FIGURE 16.19). This flow of electron density causes the O—H bond to become more polarized; as a result, water molecules bound to the metal ion are more acidic than those in the bulk solvent. This effect becomes more pronounced as the charge of the cation increases, which explains why 3+ cations are much more acidic than cations with smaller charges.
FIGURE 16.19). This flow of electron density causes the O—H bond to become more polarized; as a result, water molecules bound to the metal ion are more acidic than those in the bulk solvent. This effect becomes more pronounced as the charge of the cation increases, which explains why 3+ cations are much more acidic than cations with smaller charges.
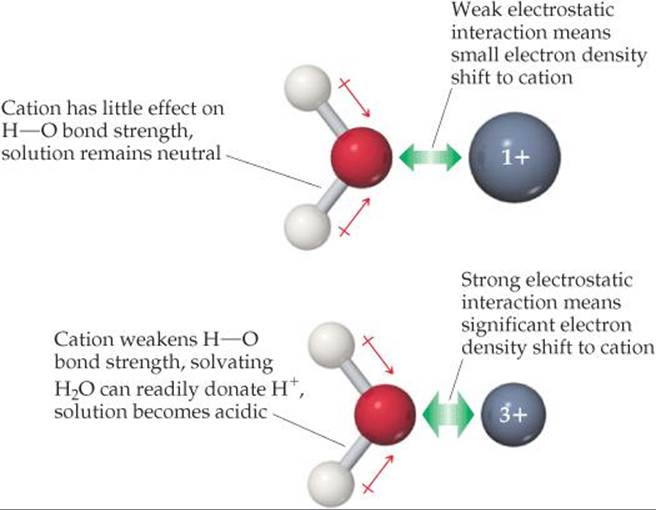
![]() FIGURE 16.19 The acidity of a hydrated cation depends on cation charge.
FIGURE 16.19 The acidity of a hydrated cation depends on cation charge.
SAMPLE INTEGRATIVE EXERCISE Putting Concepts Together
Phosphorous acid (H3PO3) has the Lewis structure
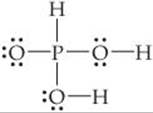
(a) Explain why H3PO3 is diprotic and not triprotic. (b) A 25.0-mL sample of an H3PO3 solution titrated with 0.102 M NaOH requires 23.3 mL of NaOH to neutralize both acidic protons. What is the molarity of the H3PO3 solution? (c) The original solution from part (b) has a pH of 1.59. Calculate the percent ionization and Ka1 for H3PO3, assuming that ![]() . (d) How does the osmotic pressure of a 0.050 M solution of HCl compare qualitatively with that of a 0.050 M solution of H3PO3? Explain.
. (d) How does the osmotic pressure of a 0.050 M solution of HCl compare qualitatively with that of a 0.050 M solution of H3PO3? Explain.
SOLUTION
We will use what we have learned about molecular structure and its impact on acidic behavior to answer part (a). We will then use stoichiometry and the relationship between pH and [H+] to answer parts (b) and (c). Finally, we will consider percent ionization in order to compare the osmotic pressure of the two solutions in part (d).
(a) Acids have polar H—X bonds. From Figure 8.7 we see that the electronegativity of H is 2.1 and that of P is also 2.1. Because the two elements have the same electronegativity, the H—P bond is nonpolar. ![]() (Section 8.4) Thus, this H cannot be acidic. The other two H atoms, however, are bonded to O, which has an electronegativity of 3.5. The H—O bonds are, therefore, polar with H having a partial positive charge. These two H atoms are consequently acidic.
(Section 8.4) Thus, this H cannot be acidic. The other two H atoms, however, are bonded to O, which has an electronegativity of 3.5. The H—O bonds are, therefore, polar with H having a partial positive charge. These two H atoms are consequently acidic.
(b) The chemical equation for the neutralization reaction is
![]()
From the definition of molarity, M = mol/L, we see that moles = M × L. ![]() (Section 4.5) Thus, the number of moles of NaOH added to the solution is
(Section 4.5) Thus, the number of moles of NaOH added to the solution is
![]()
The balanced equation indicates that 2 mol of NaOH is consumed for each mole of H3PO3. Thus, the number of moles of H3PO3 in the sample is
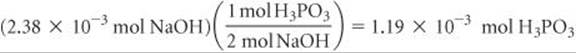
The concentration of the H3PO3 solution, therefore, equals (1.19 × 10–3mol)/(0.0250L) = 0.0476M.
(c) From the pH of the solution, 1.59, we can calculate [H+] at equilibrium:
![]()
Because ![]() , the vast majority of the ions in solution are from the first ionization step of the acid.
, the vast majority of the ions in solution are from the first ionization step of the acid.
![]()
Because one H2PO3– ion forms for each H+ ion formed, the equilibrium concentrations of H+ and H2PO3– are equal: [H+] = [H2PO3–] = 0.026 M. The equilibrium concentration of H3PO3 equals the initial concentration minus the amount that ionizes to form H+ and H2PO3–: [H3PO3] = 0.0476 M – 0.026 M = 0.022 M (two significant figures). These results can be tabulated as follows:
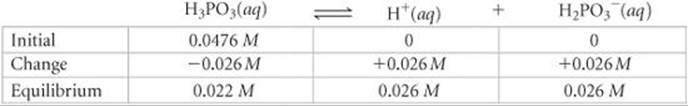
The percent ionization is
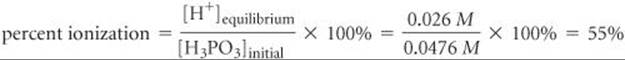
The first acid-dissociation constant is
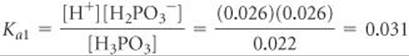
(d) Osmotic pressure is a colligative property and depends on the total concentration of particles in solution. ![]() (Section 13.5) Because HCl is a strong acid, a 0.050 M solution will contain 0.050 M H+(aq) and 0.050 M Cl– (aq), or a total of 0.100 mol/L of particles. Because H3PO3 is a weak acid, it ionizes to a lesser extent than HCl and, hence, there are fewer particles in the H3PO3 solution. As a result, the H3PO3 solution will have the lower osmotic pressure.
(Section 13.5) Because HCl is a strong acid, a 0.050 M solution will contain 0.050 M H+(aq) and 0.050 M Cl– (aq), or a total of 0.100 mol/L of particles. Because H3PO3 is a weak acid, it ionizes to a lesser extent than HCl and, hence, there are fewer particles in the H3PO3 solution. As a result, the H3PO3 solution will have the lower osmotic pressure.