CHEMISTRY THE CENTRAL SCIENCE
23 TRANSITION METALS AND COORDINATION CHEMISTRY
23.4 NOMENCLATURE AND ISOMERISM IN COORDINATION CHEMISTRY
When complexes were first discovered, they were named after the chemist who originally prepared them. A few of these names persist, as, for example, with the dark red substance NH4[Cr(NH3)2(NCS)4], which is still known as Reinecke's salt. Once the structures of complexes were more fully understood, it became possible to name them in a more systematic manner. Let's use two substances to illustrate how coordination compounds are named:
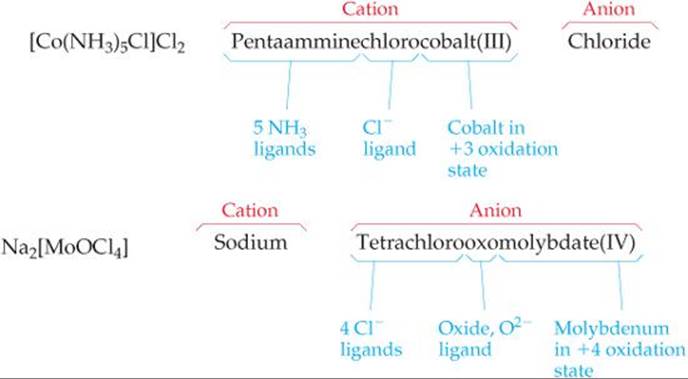
1. In naming complexes that are salts, the name of the cation is given before the name of the anion. Thus, in [Co(NH3)5Cl]Cl2 we name the [Co(NH3)5Cl]2+ cation and then the Cl–.
2. In naming complex ions or molecules, the ligands are named before the metal. Ligands are listed in alphabetical order, regardless of their charges. Prefixes that give the number of ligands are not considered part of the ligand name in determining alphabetical order. Thus, the [Co(NH3)5Cl]2+ ion is pentaamminechlorocobalt(III). (Be careful to note, however, that the metal is written first in the chemical formula.)
TABLE 23.5 • Some Common Ligands and Their Names
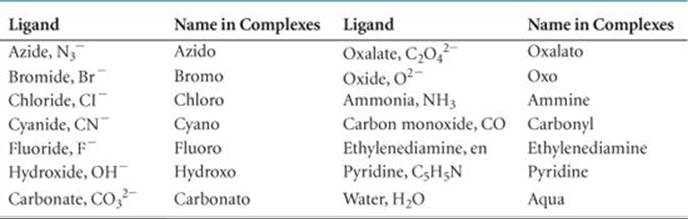
3. The names of anionic ligands end in the letter o, but electrically neutral ligands ordinarily bear the name of the molecules (![]() TABLE 23.5). Special names are used for H2O (aqua), NH3 (ammine), and CO (carbonyl). For example, [Fe(CN)2(NH3)2(H2O)2]+ is the diamminediaquadicyanoiron(III) ion.
TABLE 23.5). Special names are used for H2O (aqua), NH3 (ammine), and CO (carbonyl). For example, [Fe(CN)2(NH3)2(H2O)2]+ is the diamminediaquadicyanoiron(III) ion.
4. Greek prefixes (di-, tri-, tetra-, penta-, hexa-) are used to indicate the number of each kind of ligand when more than one is present. If the ligand contains a Greek prefix (for example, ethylenediamine) or is polydentate, the alternate prefixes bis-, tris-, tetrakis-, pentakis-, and hexakis- are used and the ligand name is placed in parentheses. For example, the name for [Co(en)3]Br3 is tris(ethylenediamine)-cobalt(III) bromide.
5. If the complex is an anion, its name ends in -ate. The compound K4[Fe(CN)6] is potassium hexacyanoferrate(II), for example, and the ion [CoCl4]2– is tetrachlorocobaltate(II) ion.
6. The oxidation number of the metal is given in parentheses in Roman numerals following the name of the metal.
Three examples for applying these rules are
[Ni(NH3)6]Br2 Hexaamminenickel(II) bromide
[Co(en)2(H20)(CN)]Cl2 Aquacyanobis (ethylenediamine) cobalt (III) chloride
Na2[MoOCl4] Sodium tetrachlorooxomolybdate(IV)
SAMPLE EXERCISE 23.4 Naming Coordination Compounds
Name the compounds (a) [Cr(H2O)4Cl2]Cl, (b) K4[Ni(CN)4].
SOLUTION
Analyze We are given the chemical formulas for two coordination compounds and assigned the task of naming them.
Plan To name the complexes, we need to determine the ligands in the complexes, the names of the ligands, and the oxidation state of the metal ion. We then put the information together following the rules listed in the text.
Solve
(a) The ligands are four water molecules—tetraaqua—and two chloride ions—dichloro. By assigning all the oxidation numbers we know for this molecule, we see that the oxidation number of Cr is +3:
Thus, we have chromium(III). Finally, the anion is chloride. The name of the compound is 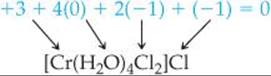 tetraaquadichlorochromium(III) chloride
tetraaquadichlorochromium(III) chloride
(b) The complex has four cyanide ion ligands, CN–, which means tetracyano, and the oxidation state of the nickel is zero:
Because the complex is an anion, the metal is indicated as nickelate(0) Putting these parts together and naming the cation first, we have 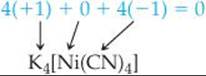 potassium tetracyanonickelate(0)
potassium tetracyanonickelate(0)
PRACTICE EXERCISE
Name the compounds (a) [Mo(NH3)3Br3]NO3, (b) (NH4)2[CuBr4]. (c) Write the formula for sodium diaquabis (oxalato) ruthenate (III).
Answers: (a) triamminetribromomolybdenum(IV) nitrate, (b) ammonium tetrabromocuprate(II) (c) Na[Ru(H2O)2(C2O4)2]
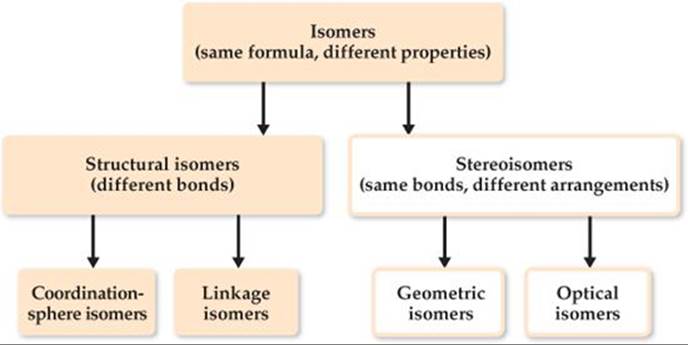
![]() FIGURE 23.19 Forms of isomerism in coordination compounds.
FIGURE 23.19 Forms of isomerism in coordination compounds.
Isomerism
When two or more compounds have the same composition but a different arrangement of atoms, we call them isomers. ![]() (Section 2.9) Here we consider two main kinds of isomers in coordination compounds: structural isomers (which have different bonds) and stereoisomers (which have the same bonds but different ways in which the ligands occupy the space around the metal center). Each of these classes also has subclasses, as shown in
(Section 2.9) Here we consider two main kinds of isomers in coordination compounds: structural isomers (which have different bonds) and stereoisomers (which have the same bonds but different ways in which the ligands occupy the space around the metal center). Each of these classes also has subclasses, as shown in ![]() FIGURE 23.19.
FIGURE 23.19.
Structural Isomerism
Many types of structural isomerism are known in coordination chemistry, including the two named in Figure 23.19: linkage isomerism and coordination-sphere isomerism. Linkage isomerism is a relatively rare but interesting type that arises when a particular ligand is capable of coordinating to a metal in two ways. The nitrite ion, NO2–, for example, can coordinate to a metal ion through either its nitrogen or one of its oxygens (![]() FIGURE 23.20). When it coordinates through the nitrogen atom, the NO2– ligand is called nitro; when it coordinates through the oxygen atom, it is called nitrito and is generally written ONO–. The isomers shown in Figure 23.20 have different properties. The nitro isomer is yellow, for example, whereas the nitrito isomer is red.
FIGURE 23.20). When it coordinates through the nitrogen atom, the NO2– ligand is called nitro; when it coordinates through the oxygen atom, it is called nitrito and is generally written ONO–. The isomers shown in Figure 23.20 have different properties. The nitro isomer is yellow, for example, whereas the nitrito isomer is red.
Another ligand capable of coordinating through either of two donor atoms is thiocyanate, SCN–, whose potential donor atoms are N and S.
Coordination-sphere isomers are isomers that differ in which species in the complex are ligands and which are outside the coordination sphere in the solid lattice. For example, three isomers have the formula CrCl3(H2O)6. When the ligands are six H2O and the chloride ions are in the crystal lattice (as counterions), we have the violet compound [Cr(H2O)6]Cl3. When the ligands are five H2O and one Cl–, with the sixth H2O and the two Cl– out in the lattice, we have the green compound [Cr(H2O)5Cl]Cl2 • H2O. The third isomer, [Cr(H2O)4Cl2]Cl • 2 H2O, is also a green compound. In the two green compounds, either one or two water molecules have been displaced from the coordination sphere by chloride ions and occupy a site in the crystal lattice.
![]() GIVE IT SOME THOUGHT
GIVE IT SOME THOUGHT
Can the ammonia ligand engage in linkage isomerism? Explain.
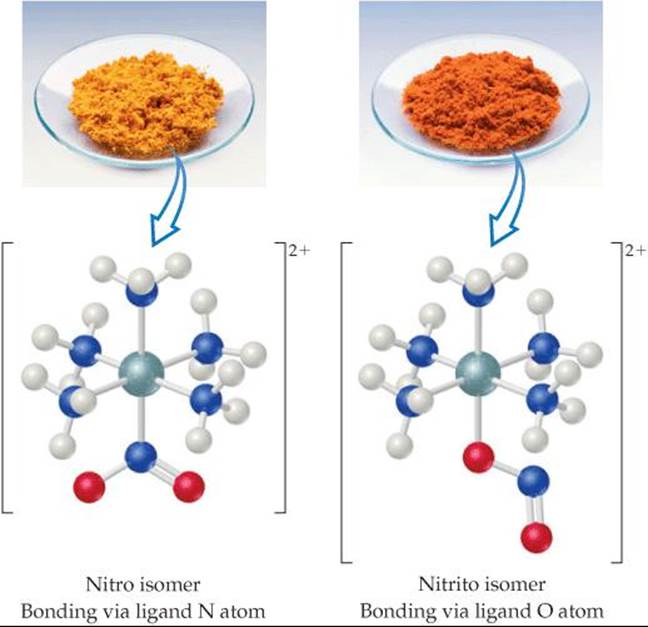
![]() FIGURE 23.20 Linkage isomerism.
FIGURE 23.20 Linkage isomerism.
![]() GO FIGURE
GO FIGURE
Which of these isomers has a nonzero dipole moment?
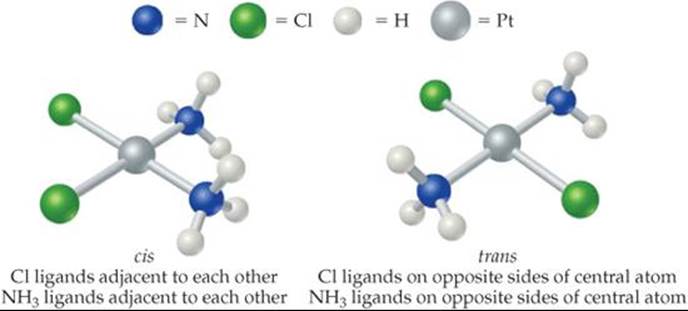
![]() FIGURE 23.21 Geometric isomerism.
FIGURE 23.21 Geometric isomerism.
Stereoisomerism
Stereoisomers have the same chemical bonds but different spatial arrangements. In the square-planar complex [Pt(NH3)2Cl2], for example, the chloro ligands can be either adjacent to or opposite each other (![]() FIGURE 23.21). (We saw an earlier example of this type of isomerism in the cobalt complex of Figure 23.7, and we'll return to that complex in a moment.) This form of stereoisomerism, in which the arrangement of the atoms is different but the same bonds are present, is called geometric isomerism. The isomer on the left in Figure 23.21, with like ligands in adjacent positions, is the cis isomer, and the isomer on the right, with like ligands across from one another, is the trans isomer.
FIGURE 23.21). (We saw an earlier example of this type of isomerism in the cobalt complex of Figure 23.7, and we'll return to that complex in a moment.) This form of stereoisomerism, in which the arrangement of the atoms is different but the same bonds are present, is called geometric isomerism. The isomer on the left in Figure 23.21, with like ligands in adjacent positions, is the cis isomer, and the isomer on the right, with like ligands across from one another, is the trans isomer.
Geometric isomers generally have different physical properties and may also have markedly different chemical reactivities. For example, cis-[Pt(NH3)2Cl2], also called cisplatin, is effective in the treatment of testicular, ovarian, and certain other cancers, whereas the trans isomer is ineffective. This is because cisplatin forms a chelate with two nitrogens of DNA, displacing the chloride ligands. The chloride ligands of the trans isomer are too far apart to form the N-Pt-N chelate with DNA nitrogens.
Geometric isomerism is also possible in octahedral complexes when two or more different ligands are present, as in the cis and trans tetraamminedichlorocobalt(III) ion in Figure 23.7. Because all the corners of a tetrahedron are adjacent to one another, cis-trans isomerism is not observed in tetrahedral complexes.
SAMPLE EXERCISE 23.5 Determining the Number of Geometric Isomers
The Lewis structure:![]() indicates that the CO molecule has two lone pairs of electrons. When CO binds to a transition-metal atom, it nearly always does so by using the C lone pair. How many geometric isomers are there for tetracarbonyldichloroiron(II)?
indicates that the CO molecule has two lone pairs of electrons. When CO binds to a transition-metal atom, it nearly always does so by using the C lone pair. How many geometric isomers are there for tetracarbonyldichloroiron(II)?
SOLUTION
Analyze We are given the name of a complex containing only monodentate ligands, and we must determine the number of isomers the complex can form.
Plan We can count the number of ligands to determine the coordination number of the Fe and then use the coordination number to predict the geometry. We can then either make a series of drawings with ligands in different positions to determine the number of isomers or deduce the number of isomers by analogy to cases we have discussed.
Solve The name indicates that the complex has four carbonyl (CO) ligands and two chloro (Cl–) ligands, so its formula is Fe(CO)4Cl2. The complex therefore has a coordination number of 6, and we can assume an octahedral geometry. Like [Co(NH3)4Cl2]+ (Figure 23.7), it has four ligands of one type and two of another. Consequently, there are two isomers possible: one with the Cl– ligands across the metal from each other, trans-[Fe(CO)4Cl2], and one with the Cl– ligands adjacent to each other, cis-[Fe(CO)4Cl2].
Comment It is easy to overestimate the number of geometric isomers. Sometimes different orientations of a single isomer are incorrectly thought to be different isomers. If two structures can be rotated so that they are equivalent, they are not isomers of each other. The problem of identifying isomers is compounded by the difficulty we often have in visualizing three-dimensional molecules from their two-dimensional representations. It is sometimes easier to determine the number of isomers if we use three-dimensional models.
PRACTICE EXERCISE
How many isomers exist for the square-planar molecule [Pt(NH3)2ClBr]?
Answer: two
The second type of stereoisomerism listed in Figure 23.19 is optical isomerism. Optical isomers, called enantiomers, are mirror images that cannot be superimposed on each other. They bear the same resemblance to each other that your left hand bears to your right hand. If you look at your left hand in a mirror, the image is identical to your right hand (![]() FIGURE 23.22). No matter how hard you try, however, you cannot superimpose your two hands on each other. An example of a complex that exhibits this type of isomerism is the [Co(en)3]3+ ion. Figure 23.22 shows the two enantiomers of this complex and their mirror-image relationship. Just as there is no way that we can twist or turn our right hand to make it look identical to our left, so also there is no way to rotate one of these enantiomers to make it identical to the other. Molecules or ions that are not superimposable on their mirror image are said to be chiral (pronounced KY-rul).
FIGURE 23.22). No matter how hard you try, however, you cannot superimpose your two hands on each other. An example of a complex that exhibits this type of isomerism is the [Co(en)3]3+ ion. Figure 23.22 shows the two enantiomers of this complex and their mirror-image relationship. Just as there is no way that we can twist or turn our right hand to make it look identical to our left, so also there is no way to rotate one of these enantiomers to make it identical to the other. Molecules or ions that are not superimposable on their mirror image are said to be chiral (pronounced KY-rul).
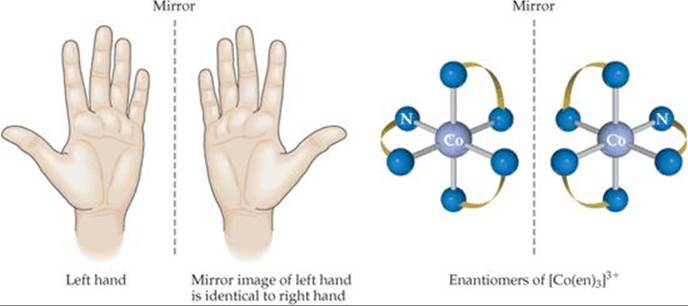
![]() FIGURE 23.22 Optical isomerism.
FIGURE 23.22 Optical isomerism.
SAMPLE EXERCISE 23.6 Predicting Whether a Complex Has Optical Isomers
Does either cis-[Co(en)2Cl2]+ or trans-[Co(en)2Cl2]– have optical isomers?
SOLUTION
Analyze We are given the chemical formula for two geometric isomers and asked to determine whether either one has optical isomers. Because en is a bidentate ligand, we know that both complexes are octahedral and both have coordination number 6.
Plan We need to sketch the structures of the cis and trans isomers and their mirror images. We can draw the en ligand as two N atoms connected by an arc. If the mirror image cannot be superimposed on the original structure, the complex and its mirror image are optical isomers.
Solve The trans isomer of [Co(en)2Cl2]+ and its mirror image are:
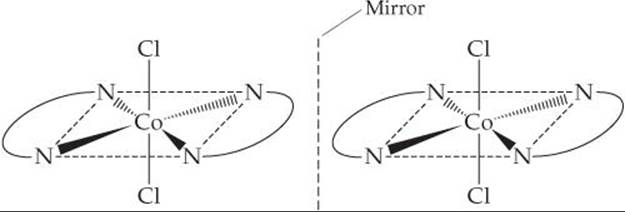
Notice that the mirror image of the isomer is identical to the original. Consequently trans-[Co(en)2Cl2]+ does not exhibit optical isomerism.
The mirror image of the cis isomer cannot be superimposed on the original:
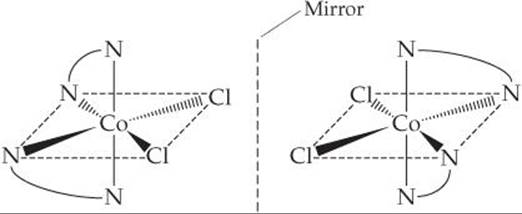
Thus, the two cis structures are optical isomers (enantiomers). We say that cis-[Co(en)2Cl2]+ is a chiral complex.
PRACTICE EXERCISE
Does the square-planar complex ion [Pt(NH3)(N3)ClBr]– have optical isomers? Explain your answer.
Answer: no, because the complex is flat. This complex ion does, however, have geometric isomers (for example, the Cl and Br ligands could be cis or trans).
The properties of two optical isomers differ only if the isomers are in a chiral environment—that is, an environment in which there is a sense of right- and left-handedness. A chiral enzyme, for example, might catalyze the reaction of one optical isomer but not the other. Consequently, one optical isomer may produce a specific physiological effect in the body, with its mirror image producing either a different effect or none at all. Chiral reactions are also extremely important in the synthesis of pharmaceuticals and other industrially important chemicals.
Optical isomers are usually distinguished from each other by their interaction with plane-polarized light. If light is polarized—for example, by being passed through a sheet of polarizing film—the electric-field vector of the light is confined to a single plane (![]() FIGURE 23.23). If the polarized light is then passed through a solution containing one optical isomer, the plane of polarization is rotated either to the right or to the left. The isomer that rotates the plane of polarization to the right is dextrorotatory; it is the dextro, or d, isomer (Latin dexter, “right”). Its mirror image rotates the plane of polarization to the left; it is levorotatory and is the levo, or l, isomer (Latin laevus, “left”). The [Co(en)3]3+ isomer on the right in Figure 23.22 is found experimentally to be the l isomer of this ion. Its mirror image is the d isomer. Because of their effect on plane-polarized light, chiral molecules are said to be optically active.
FIGURE 23.23). If the polarized light is then passed through a solution containing one optical isomer, the plane of polarization is rotated either to the right or to the left. The isomer that rotates the plane of polarization to the right is dextrorotatory; it is the dextro, or d, isomer (Latin dexter, “right”). Its mirror image rotates the plane of polarization to the left; it is levorotatory and is the levo, or l, isomer (Latin laevus, “left”). The [Co(en)3]3+ isomer on the right in Figure 23.22 is found experimentally to be the l isomer of this ion. Its mirror image is the d isomer. Because of their effect on plane-polarized light, chiral molecules are said to be optically active.
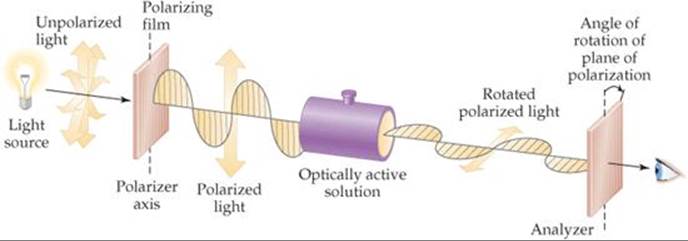
![]() FIGURE 23.23 Using polarized light to detect optical activity.
FIGURE 23.23 Using polarized light to detect optical activity.
![]() GIVE IT SOME THOUGHT
GIVE IT SOME THOUGHT
What is the similarity and what is the difference between the d and l isomers of a compound?
When a substance with optical isomers is prepared in the laboratory, the chemical environment during the synthesis is not usually chiral. Consequently, equal amounts of the two isomers are obtained, and the mixture is said to be racemic. A racemic mixture does not rotate polarized light because the rotatory effects of the two isomers cancel each other.