CHEMISTRY THE CENTRAL SCIENCE
3 STOICHIOMETRY: CALCULATIONS WITH CHEMICAL FORMULAS AND EQUATIONS
3.6 QUANTITATIVE INFORMATION FROM BALANCED EQ UATIONS
The coefficients in a chemical equation represent the relative numbers of molecules in a reaction. The mole concept allows us to convert this information to the masses of the substances in the reaction. For instance, the coefficients in the balanced equation
![]()
indicate that two molecules of H2 react with one molecule of O2 to form two molecules of H2O. It follows that the relative numbers of moles are identical to the relative numbers of molecules:
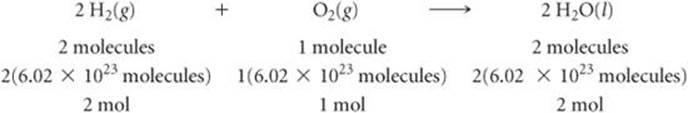
We can generalize this observation to all balanced chemical equations: The coefficients in a balanced chemical equation indicate both the relative numbers of molecules (or formula units) in the reaction and the relative numbers of moles. ![]() FIGURE 3.15 shows how this result corresponds to the law of conservation of mass.
FIGURE 3.15 shows how this result corresponds to the law of conservation of mass.
The quantities 2 mol H2, 1 mol O2, and 2 mol H2O given by the coefficients in Equation 3.12 are called stoichiometrically equivalent quantities. The relationship between these quantities can be represented as
![]()
where the ![]() symbol means “is stoichiometrically equivalent to.” Stoichiometric relations such as these can be used to convert between quantities of reactants and products in a chemical reaction. For example, the number of moles of H2O produced from 1.57 mol of O2 is
symbol means “is stoichiometrically equivalent to.” Stoichiometric relations such as these can be used to convert between quantities of reactants and products in a chemical reaction. For example, the number of moles of H2O produced from 1.57 mol of O2 is
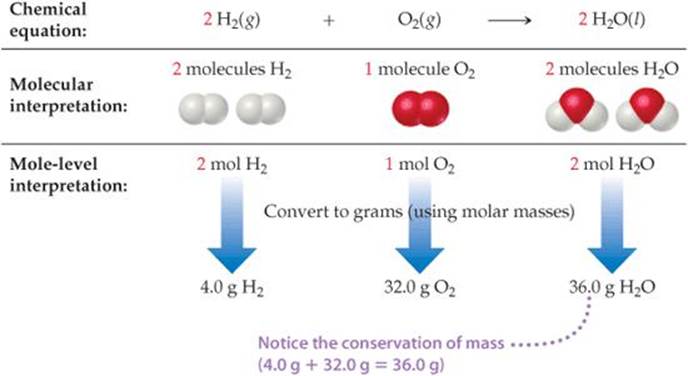
![]() FIGURE 3.15 Interpreting a balanced chemical equation quantitatively.
FIGURE 3.15 Interpreting a balanced chemical equation quantitatively.
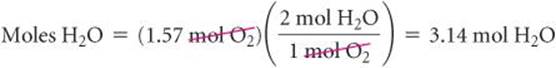
![]() GIVE IT SOME THOUGHT
GIVE IT SOME THOUGHT
When 1.57 mol O2 reacts with H2 to form H2O, how many moles of H2 are consumed in the process?
As an additional example, consider the combustion of butane (C4H10), the fuel in disposable general-purpose lighters:
![]()
Let's calculate the mass of CO2 produced when 1.00 g of C4H10 is burned. The coefficients in Equation 3.13 tell us how the amount of C4H10 consumed is related to the amount of CO2 produced: 2 mol C4H10![]() 8 mol CO2. To use this stoichiometric relationship, we must convert grams of C4H10 to moles using the molar mass of C4H10, 58.0 g/mol:
8 mol CO2. To use this stoichiometric relationship, we must convert grams of C4H10 to moles using the molar mass of C4H10, 58.0 g/mol:
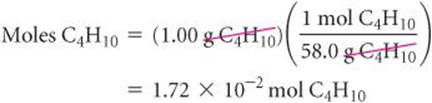
We then use the stoichiometric factor from the balanced equation to calculate moles of CO2:
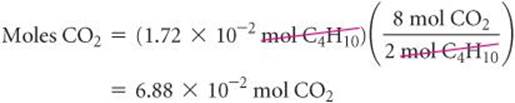
Finally, we use the molar mass of CO2, 44.0 g/mol, to calculate the CO2 mass in grams:
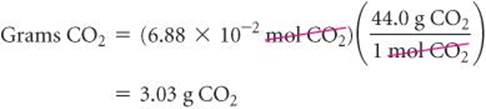
This conversion sequence involves three steps, as illustrated in ![]() FIGURE 3.16. These three conversions can be combined in a single equation:
FIGURE 3.16. These three conversions can be combined in a single equation:
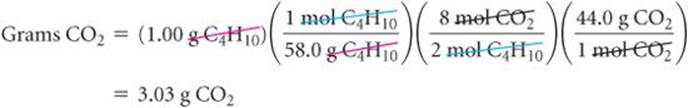
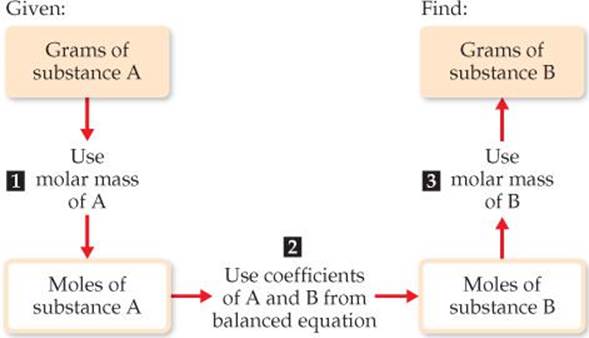
![]() FIGURE 3.16 Procedure for calculating amounts of reactants consumed or products formed in a reaction. The number of grams of a reactant consumed or product formed can be calculated in three steps, starting with the number of grams of any reactant or product. Notice how molar masses and the coefficients in the balanced equation are used.
FIGURE 3.16 Procedure for calculating amounts of reactants consumed or products formed in a reaction. The number of grams of a reactant consumed or product formed can be calculated in three steps, starting with the number of grams of any reactant or product. Notice how molar masses and the coefficients in the balanced equation are used.
To calculate the amount of O2 consumed in the reaction of Equation 3.13, we again rely on the coefficients in the balanced equation for our stoichiometric factor: 2 mol C4H10![]() 13 mol O2:
13 mol O2:
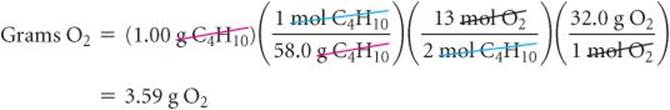
![]() GIVE IT SOME THOUGHT
GIVE IT SOME THOUGHT
If 20.00 g of a compound reacts completely with 30.00 g of another compound in a combination reaction, how many grams of product are formed?
SAMPLE EXERCISE 3.16 Calculating Amounts of Reactants and Products
Determine how many grams of water are produced in the oxidation of 1.00 g of glucose, C6H12O6:
C6H12O6(s) + 6 O2(g) → 6 CO2(g) + 6 H2O(l)
SOLUTION
Analyze We are given the mass of a reactant and must determine the mass of a product in the given reaction.
Plan The general strategy, as outlined in Figure 3.16, requires three steps:
1. Convert grams of C6H12O6 to moles using the molar mass of C6H12O6.
2. Convert moles of C6H12O6 to moles of H2O using the stoichiometric relationship 1 mol C6H12O6![]() 6 mol H2O.
6 mol H2O.
3. Convert moles of H2O to grams using the molar mass of H2O.
Solve
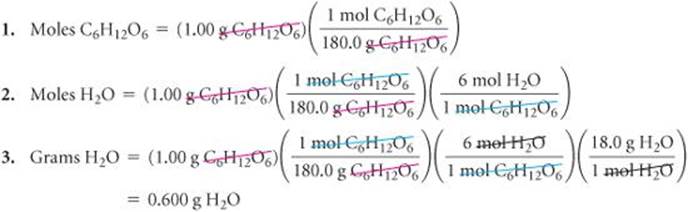
The steps can be summarized in a diagram like that in Figure 3.16:
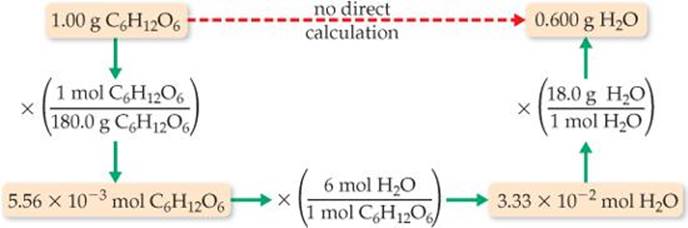
Check We can check how reasonable our result is by doing a ballpark estimate of the mass of H2O. Because the molar mass of glucose is 180 g/mol, 1 gram of glucose equals 1/180 mol. Because one mole of glucose yields 6 mol H2O, we would have 6/180 = 1/30 mol H2O. The molar mass of water is 18 g/mol, so we have 1/30 × 18 = 6/10 = 0.6 g of H2O, which agrees with the full calculation. The units, grams H2O, are correct. The initial data had three significant figures, so three significant figures for the answer is correct.
Comment An average adult ingests 2 L of water daily and eliminates 2.4 L. The “extra” 0.4 L is produced in the metabolism of foodstuffs, such as oxidation of glucose. The desert rat (kangaroo rat), on the other hand, apparently never drinks water. It survives on its metabolic water.
PRACTICE EXERCISE
Decomposition of KClO3 is sometimes used to prepare small amounts of O2 in the laboratory: 2 KClO3(s) → 2 KCl(s) + 3 O2(g). How many grams of O2 can be prepared from 4.50 g of KClO3?
Answer: 1.77 g
SAMPLE EXERCISE 3.17 Calculating Amounts of Reactants and Products
Solid lithium hydroxide is used in space vehicles to remove the carbon dioxide gas exhaled by astronauts. The hydroxide reacts with the carbon dioxide to form solid lithium carbonate and liquid water. How many grams of carbon dioxide can be absorbed by 1.00 g of lithium hydroxide?
SOLUTION
Analyze We are given a verbal description of a reaction and asked to calculate the number of grams of one reactant that reacts with 1.00 g of another.
Plan The verbal description of the reaction can be used to write a balanced equation:
2 LiOH(s) + CO2(g) → Li2CO3(s) + H2O(l)
We are given the mass in grams of LiOH and asked to calculate the mass in grams of CO2. We can accomplish this task by using the three conversion steps in Figure 3.16. The conversion of step 1 requires the molar mass of LiOH (6.94 + 16.00 + 1.01 = 23.95 g/mol). The conversion of step 2 is based on a stoichiometric relationship from the balanced chemical equation: 2 mol LiOH ![]() 1 mol CO2. For the step 3 conversion, we use the molar mass of CO2: 12.01 + 2(16.00) = 44.01 g/mol.
1 mol CO2. For the step 3 conversion, we use the molar mass of CO2: 12.01 + 2(16.00) = 44.01 g/mol.
Solve
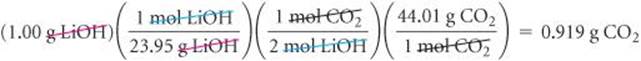
Check Notice that 23.95 g LiOH/mol ≈ 24 g LiOH/mol, that 24 g LiOH/mol × 2 mol LiOH = 48 g LiOH, and (44 g CO2/mol)/(48 g LiOH) is slightly less than 1. Thus, the magnitude of our answer, 0.919 g CO2, is reasonable based on the amount of starting LiOH. The significant figures and units are also appropriate.
PRACTICE EXERCISE
Propane, C3H8 (Figure 3.8), is a common fuel used for cooking and home heating. What mass of O2 is consumed in the combustion of 1.00 g of propane?
Answer: 3.64 g