CHEMISTRY THE CENTRAL SCIENCE
7 PERIODIC PROPERTIES OF THE ELEMENTS
7.7 TRENDS FOR GROUP 1A AND GROUP 2A METALS
As we have seen, elements in a given group possess general similarities. However, trends also exist within each group. In this section we use the periodic table and our knowledge of electron configurations to examine the chemistry of the alkali metals and alkaline earth metals.

![]() FIGURE 7.18 Elemental silicon.
FIGURE 7.18 Elemental silicon.
Although it looks metallic, silicon, a metalloid, is brittle and a poor thermal and electrical conductor.
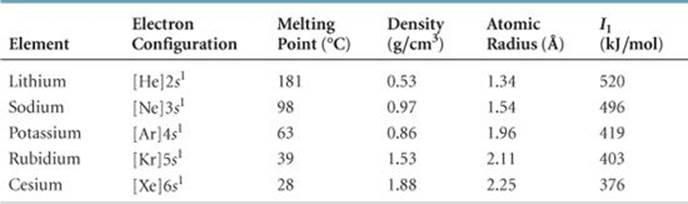
TABLE 7.4 • Some Properties of the Alkali Metals
Group 1A: The Alkali Metals
The alkali metals are soft metallic solids (![]() FIGURE 7.19). All have characteristic metallic properties, such as a silvery, metallic luster and high thermal and electrical conductivity. The name alkali comes from an Arabic word meaning “ashes.” Many compounds of sodium and potassium, two alkali metals, were isolated from wood ashes by early chemists.
FIGURE 7.19). All have characteristic metallic properties, such as a silvery, metallic luster and high thermal and electrical conductivity. The name alkali comes from an Arabic word meaning “ashes.” Many compounds of sodium and potassium, two alkali metals, were isolated from wood ashes by early chemists.
As ![]() TABLE 7.4 shows, the alkali metals have low densities and melting points, and these properties vary in a fairly regular way with increasing atomic number. We see the usual trends as we move down the group, such as increasing atomic radius and decreasing first ionization energy. The alkali metal of any given period has the lowest I 1 value in the period (Figure 7.9), which reflects the relative ease with which its outer s electron can be removed. As a result, the alkali metals are all very reactive, readily losing one electron to form ions carrying a 1+ charge.
TABLE 7.4 shows, the alkali metals have low densities and melting points, and these properties vary in a fairly regular way with increasing atomic number. We see the usual trends as we move down the group, such as increasing atomic radius and decreasing first ionization energy. The alkali metal of any given period has the lowest I 1 value in the period (Figure 7.9), which reflects the relative ease with which its outer s electron can be removed. As a result, the alkali metals are all very reactive, readily losing one electron to form ions carrying a 1+ charge. ![]() (Section 2.7)
(Section 2.7)
The alkali metals exist in nature only as compounds. Sodium and potassium are relatively abundant in Earths crust, in seawater, and in biological systems, usually as the cations of ionic compounds. All alkali metals combine directly with most nonmetals. For example, they react with hydrogen to form hydrides and with sulfur to form sulfides:
![]()
![]()
where M represents any alkali metal. In hydrides of the alkali metals (LiH, NaH, and so forth), hydrogen is present as H−, the hydride ion. A hydrogen atom that has gained an electron, this ion is distinct from the hydrogen ion, H+, formed when a hydrogen atom loses its electron.
The alkali metals react vigorously with water, producing hydrogen gas and a solution of an alkali metal hydroxide:
![]()
These reactions are very exothermic. In many cases enough heat is generated to ignite the H2, producing a fire or sometimes even an explosion (![]() FIGURE 7.20). The reaction is most violent for the heavier alkali metals, in keeping with their lower ionization energies.
FIGURE 7.20). The reaction is most violent for the heavier alkali metals, in keeping with their lower ionization energies.

![]() FIGURE 7.19 Sodium, like the other alkali metals, is soft enough to be cut with a knife.
FIGURE 7.19 Sodium, like the other alkali metals, is soft enough to be cut with a knife.
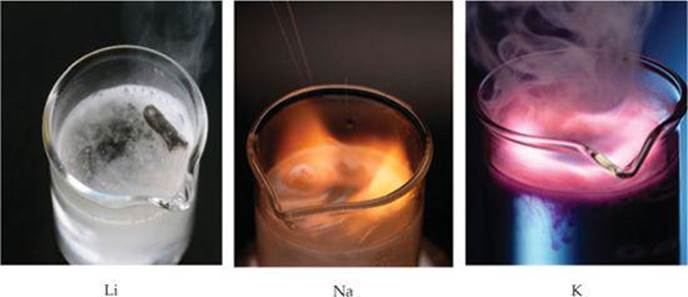
![]() FIGURE 7.20 The alkali metals react vigorously with water.
FIGURE 7.20 The alkali metals react vigorously with water.
![]() GO FIGURE
GO FIGURE
If we had potassium vapor lamps, what color would they be?

![]() FIGURE 7.22 The characteristic yellow light in a sodium lamp is the result of electrons in the high-energy 3p orbital falling back to the lower-energy 3s orbital. The energy gap corresponds to the energy of yellow light.
FIGURE 7.22 The characteristic yellow light in a sodium lamp is the result of electrons in the high-energy 3p orbital falling back to the lower-energy 3s orbital. The energy gap corresponds to the energy of yellow light.
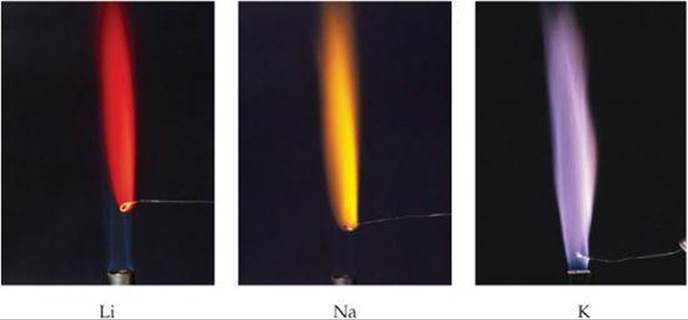
![]() FIGURE 7.21 Placed in a flame, ions of each alkali metal emit light of a characteristic wavelength.
FIGURE 7.21 Placed in a flame, ions of each alkali metal emit light of a characteristic wavelength.
The reactions between the alkali metals and oxygen are complex. Metal oxides, which contain the O2− ion, are usually formed:
![]()
When dissolved in water, Li2O and other soluble metal oxides form hydroxide ions from the reaction of O2− ions with H2O (Equation 7.10). In contrast, the other alkali metals react with oxygen to form metal peroxides, which contain the O22− ion:
![]()
Potassium, rubidium, and cesium also form compounds that contain the O2− ion, which we call the superoxide ion. For example, potassium forms potassium superox-ide, KO2:
![]()
Be aware that the reactions in Equations 7.20 and 7.21 are somewhat surprising; in most cases, the reaction of oxygen with a metal forms the metal oxide.
As is evident from Equations 7.18 through 7.21, the alkali metals are extremely reactive toward water and oxygen. Because of this, the metals are usually stored submerged in a liquid hydrocarbon, such as mineral oil or kerosene.
Although alkali metal ions are colorless, each emits a characteristic color when placed in a flame (![]() FIGURE 7.21). The ions are reduced to gaseous metal atoms in the flame. The high temperature excites the valence electron from the ground state to a higher-energy orbital, causing the atom to be in an excited state. The atom then emits energy in the form of visible light as the electron falls back into the lower-energy orbital and the atom returns to its ground state. The light emitted is at a specific wavelength for each element, just as we saw earlier for line spectra of hydrogen and sodium
FIGURE 7.21). The ions are reduced to gaseous metal atoms in the flame. The high temperature excites the valence electron from the ground state to a higher-energy orbital, causing the atom to be in an excited state. The atom then emits energy in the form of visible light as the electron falls back into the lower-energy orbital and the atom returns to its ground state. The light emitted is at a specific wavelength for each element, just as we saw earlier for line spectra of hydrogen and sodium ![]() (Section 6.3). The characteristic yellow emission of sodium at 589 nm is the basis for sodium vapor lamps (
(Section 6.3). The characteristic yellow emission of sodium at 589 nm is the basis for sodium vapor lamps (![]() FIGURE 7.22).
FIGURE 7.22).
![]() GIVE IT SOME THOUGHT
GIVE IT SOME THOUGHT
Cesium tends to be the most reactive of the stable alkali metals (francium, Fr, is radioactive and has not been extensively studied). What atomic property of Cs is most responsible for its high reactivity?
SAMPLE EXERCISE 7.10 Reactions of an Alkali Metal
Write a balanced equation for the reaction of cesium metal with (a) Cl2(g), (b) H2O(l), (c) H2(g).
SOLUTION
Analyze and Plan Because cesium is an alkali metal, we expect its chemistry to be dominated by oxidation of the metal to Cs+ ions. Further, we recognize that Cs is far down the periodic table, which means it is among the most active of all metals and probably reacts with all three substances.
Solve The reaction between Cs and Cl2 is a simple combination reaction between a metal and a nonmetal, forming the ionic compound CsCl:
![]()
From Equations 7.18 and 7.16, we predict the reactions of cesium with water and hydrogen to proceed as follows:
![]()
All three reactions are redox reactions where cesium forms a Cs+ ion. The Cl−, OH−, and H− are all 1— ions, which means the products have 1:1 stoichiometry with Cs+.
PRACTICE EXERCISE
Write a balanced equation for the reaction between potassium metal and elemental sulfur.
Answer: ![]()
 CHEMISTRY AND LIFE
CHEMISTRY AND LIFE
THE IMPROBABLE DEVELOPMENT OF LITHIUM DRUGS
Alkali metal ions tend to play an unexciting role in most chemical reactions. As noted in Section 4.2, all salts of the alkali metal ions are soluble in water, and the ions are spectators in most aqueous re-I actions (except for those involving the alkali metals in their elemental form, such as in Equations 7.16 through 7.21). However, these ions play an important role in human physiology. Sodium and potassium ions, for example, are major components of blood plasma and intra-cellular fluid, respectively, with average concentrations of 0.1 M. I These electrolytes serve as vital charge carriers in normal cellular function. In contrast, the lithium ion has no known function in normal human physiology. Since the discovery of lithium in 1817, however, people have believed that salts of the element possessed almost mystical healing powers. There were even claims that lithium ions were an ingredient in ancient “fountain of youth” formulas. In 1927, C. L. Grigg began marketing a soft drink that contained lithium. The I original unwieldy name of the beverage was “Bib-Label Lithiated I Lemon-Lime Soda,” which was soon changed to the simpler and Imore familiar name 7UP® (![]() FIGURE 7.23).
FIGURE 7.23).
Because of concerns of the Food and Drug Administration, lithium was removed from 7UP® during the early 1950s. At nearly the same time, psychiatrists discovered that the lithium ion has a remarkable therapeutic effect on the mental disorder called bipolar affective disorder, ormanic-depressive illness. Over 1 million Americans suffer from this psychosis, undergoing severe mood swings from deep depression to a manic euphoria. The lithium ion smoothes these mood swings, allowing the bipolar patient to function more effectively in daily life.
The antipsychotic action of Li+ was discovered by accident in the 1940s by Australian psychiatrist John Cade as he was researching the use of uric acid—a component of urine—to treat manicdepressive illness. He administered the acid to manic laboratory animals in the form of its most soluble salt, lithium urate, and found that many of the manic symptoms seemed to disappear. Later studies showed that uric acid has no role in the therapeutic effects observed; rather, the Li+ ions were responsible. Because lithium overdose can cause severe side effects in humans, including kidney failure and death, lithium salts were not approved as antipsychotic drugs for humans until 1970. Today Li+ is usually administered orally in the form of Li2CO3, which is the active ingredient in prescription drugs such as Eskalith®. Lithium drugs are effective for about 70% of bipolar patients who take it.
In this age of sophisticated drug design and biotechnology, the simple lithium ion is still the most effective treatment of this destructive psychological disorder. Remarkably, in spite of intensive research, scientists still do not fully understand the biochemical action of lithium that leads to its therapeutic effects. Because of its similarity to Na+, Li+ is incorporated into blood plasma, where it can affect the behavior of nerve and muscle cells. Because Li+ has a smaller radius than Na (Figure 7.7), the way Li+ interacts with molecules in human cells is different from the way Na+ interacts with the molecules. Other studies indicate that Li+ alters the function of certain neurotransmitters, which might lead to its effectiveness as an antipsychotic drug.

![]() FIGURE 7.23 Lithium no more. The soft drink 7UP® originally contained a lithium salt that was claimed to give the beverage healthful benefits, including “an abundance of energy, enthusiasm, a clear complexion, lustrous hair, and shining eyes!” The lithium was removed from the beverage in the early 1950s, about the time that the antipsychotic action of Li+ was discovered.
FIGURE 7.23 Lithium no more. The soft drink 7UP® originally contained a lithium salt that was claimed to give the beverage healthful benefits, including “an abundance of energy, enthusiasm, a clear complexion, lustrous hair, and shining eyes!” The lithium was removed from the beverage in the early 1950s, about the time that the antipsychotic action of Li+ was discovered.
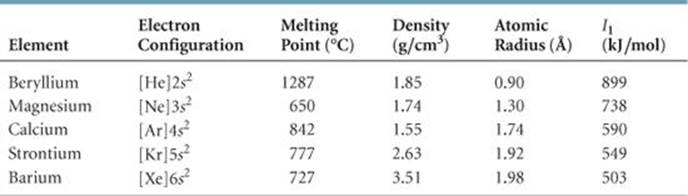
TABLE 7.5 • Some Properties of the Alkaline Earth Metals
Group 2A: The Alkaline Earth Metals
Like the alkali metals, the alkaline earth metals are all solids at room temperature and have typical metallic properties (![]() TABLE 7.5). Compared with the alkali metals, the alkaline earth metals are harder and more dense and melt at higher temperatures.
TABLE 7.5). Compared with the alkali metals, the alkaline earth metals are harder and more dense and melt at higher temperatures.
The first ionization energies of the alkaline earth metals are low but not as low as those of the alkali metals. Consequently, the alkaline earth metals are less reactive than their alkali metal neighbors. As noted in Section 7.4, the ease with which the elements lose electrons decreases as we move across a period and increases as we move down a group. Thus, beryllium and magnesium, the lightest alkaline earth metals, are the least reactive.
The trend of increasing reactivity within the group is shown by the way the alkaline earth metals behave in the presence of water. Beryllium does not react with either water or steam, even when heated red-hot. Magnesium reacts slowly with liquid water and more readily with steam:
![]()
Calcium and the elements below it react readily with water at room temperature (although more slowly than the alkali metals adjacent to them in the periodic table). The reaction between calcium and water (![]() FIGURE 7.24), for example, is
FIGURE 7.24), for example, is
![]()
Equations 7.22 and 7.23 illustrate the dominant pattern in the reactivity of the alkaline earth elements: They tend to lose their two outer s electrons and form 2+ ions. For example, magnesium reacts with chlorine at room temperature to form MgCl2 and burns with dazzling brilliance in air to give MgO:
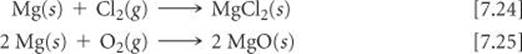
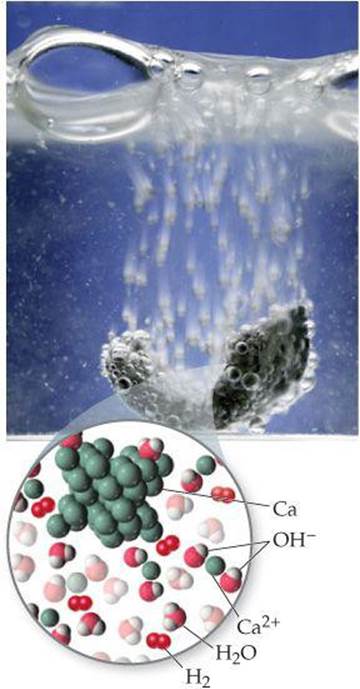
![]() FIGURE 7.24 Elemental calcium reacts with water.
FIGURE 7.24 Elemental calcium reacts with water.
In the presence of O2, magnesium metal is protected by a thin coating of water-insoluble MgO. Thus, even though Mg is high in the activity series ![]() (Section 4.4), it can be incorporated into lightweight structural alloys used in, for example, automobile wheels. The heavier alkaline earth metals (Ca, Sr, and Ba) are even more reactive toward nonmetals than is magnesium.
(Section 4.4), it can be incorporated into lightweight structural alloys used in, for example, automobile wheels. The heavier alkaline earth metals (Ca, Sr, and Ba) are even more reactive toward nonmetals than is magnesium.
The heavier alkaline earth ions give off characteristic colors when heated in a hot flame. Strontium salts produce the brilliant red color in fireworks, and barium salts produce the green color.
Like their neighbors sodium and potassium, magnesium and calcium are relatively abundant on Earth and in seawater and are essential for living organisms as cations in ionic compounds. Calcium is particularly important for growth and maintenance of bones and teeth.
![]() GIVE IT SOME THOUGHT
GIVE IT SOME THOUGHT
Calcium carbonate, CaCO3, is often used as a dietary calcium supplement for bone health. Although CaCO3(s) is insoluble in water (Table 4.1), it can be taken orally to allow for the delivery of Ca2+(aq) ions to the musculoskeletal system. Why is this the case? [Hint: Recall the reactions of metal carbonates discussed in Section 4.3.]