CHEMISTRY THE CENTRAL SCIENCE
9 MOLECULAR GEOMETRY AND BONDING THEORIES
9.5 HYBRID ORBITALS
The VSEPR model, simple as it is, does a surprisingly good job at predicting molecular shape, despite the fact that it has no obvious relationship to the filling and shapes of atomic orbitals. For example, based on the shapes and orientations of the 2s and 2p or-bitals on a carbon atom, it is not obvious why a CH4 molecule should have a tetrahedral geometry. How can we reconcile the notion that covalent bonds are formed from overlap of atomic orbitals with the molecular geometries that come from the VSEPR model?
To begin with, we recall that atomic orbitals are mathematical functions that come from the quantum mechanical model for atomic structure. ![]() (Section 6.5) To explain molecular geometries, we can assume that the atomic orbitals on an atom (usually the central atom) mix to form new orbitals called hybrid orbitals. The shape of any hybrid orbital is different from the shapes of the original atomic orbitals. The process of mixing atomic orbitals is a mathematical operation called hybridization. The total number of atomic orbitals on an atom remains constant, so the number of hybrid orbitals on an atom equals the number of atomic orbitals that are mixed.
(Section 6.5) To explain molecular geometries, we can assume that the atomic orbitals on an atom (usually the central atom) mix to form new orbitals called hybrid orbitals. The shape of any hybrid orbital is different from the shapes of the original atomic orbitals. The process of mixing atomic orbitals is a mathematical operation called hybridization. The total number of atomic orbitals on an atom remains constant, so the number of hybrid orbitals on an atom equals the number of atomic orbitals that are mixed.
As we examine the common types of hybridization, notice the connection between the type of hybridization and certain of the molecular geometries predicted by the VSEPR model: linear, bent, trigonal planar, and tetrahedral.
sp Hybrid Orbitals
To illustrate the process of hybridization, consider the BeF2 molecule, which has the Lewis structure
![]()
The VSEPR model correctly predicts that BeF2 is linear with two identical Be—F bonds. How can we use valence-bond theory to describe the bonding? The electron configuration of F (1s22s22p2) indicates an unpaired electron in a 2p orbital. This electron can be paired with an unpaired Be electron to form a polar covalent bond. Which orbitals on the Be atom, however, overlap with those on the F atoms to form the Be—F bonds?
The orbital diagram for a ground-state Be atom is
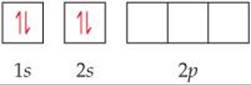
Because it has no unpaired electrons, the Be atom in its ground state cannot bond with the fluorine atoms. The Be atom could form two bonds, however, by “promoting” one of the 2s electrons to a 2p orbital:
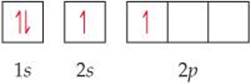
The Be atom now has two unpaired electrons and can therefore form two polar covalent bonds with F atoms. The two bonds would not be identical, however, because a Be 2s orbital would be used to form one of the bonds and a 2p orbital would be used to form the other. Therefore, although the promotion of an electron allows two Be—F bonds to form, we still have not explained the structure of BeF2.
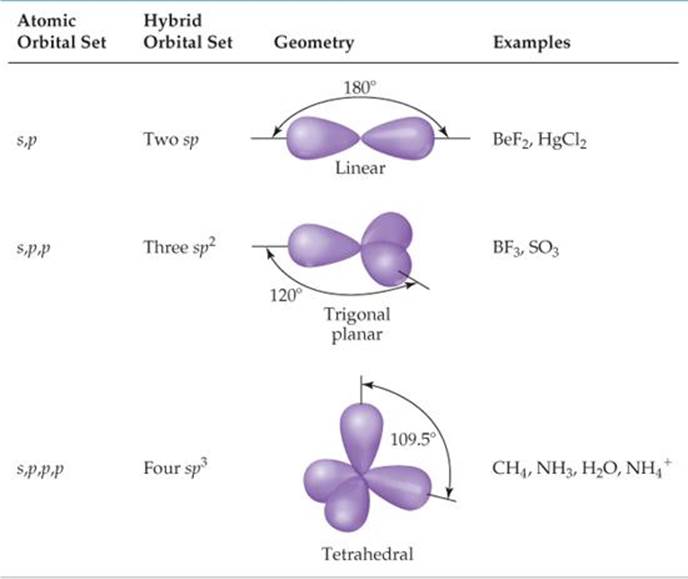
We can solve this dilemma by “mixing” the 2s orbital with one 2p orbital to generate two new orbitals, as shown in ![]() FIGURE 9.15. Like p orbitals, each new orbital has two lobes. Unlike p orbitals, however, one lobe is much larger than the other. The two new orbitals are identical in shape, but their large lobes point in opposite directions. These two new orbitals, which we color-code purple in Figure 9.15, are hybrid orbitals. Because we have hybridized one s and one p orbital, we call each hybrid an sp hybrid orbital. According to the valence-bond model, a linear arrangement of electron domains implies sp hybridization.
FIGURE 9.15. Like p orbitals, each new orbital has two lobes. Unlike p orbitals, however, one lobe is much larger than the other. The two new orbitals are identical in shape, but their large lobes point in opposite directions. These two new orbitals, which we color-code purple in Figure 9.15, are hybrid orbitals. Because we have hybridized one s and one p orbital, we call each hybrid an sp hybrid orbital. According to the valence-bond model, a linear arrangement of electron domains implies sp hybridization.
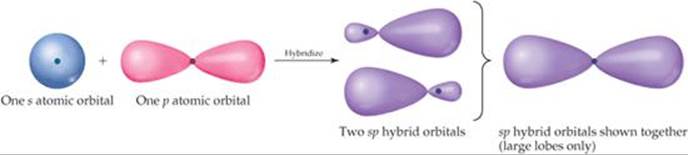
![]() FIGURE 9.15 Formation of sp hybrid orbitals.
FIGURE 9.15 Formation of sp hybrid orbitals.
For the Be atom of BeF2, we write the orbital diagram for the formation of two sp hybrid orbitals as
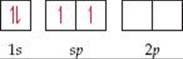
The electrons in the sp hybrid orbitals can form bonds with the two fluorine atoms (![]() FIGURE 9.16). Because the sp hybrid orbitals are equivalent but point in opposite directions, BeF2 has two identical bonds and a linear geometry. The remaining two 2p atomic orbitals of Be remain unhybridized and are vacant. Remember also that each fluorine atom has two other valence p atomic orbitals, each containing one nonbonding electron pair. Those atomic orbitals are omitted from Figure 9.16 to keep the illustration simpler.
FIGURE 9.16). Because the sp hybrid orbitals are equivalent but point in opposite directions, BeF2 has two identical bonds and a linear geometry. The remaining two 2p atomic orbitals of Be remain unhybridized and are vacant. Remember also that each fluorine atom has two other valence p atomic orbitals, each containing one nonbonding electron pair. Those atomic orbitals are omitted from Figure 9.16 to keep the illustration simpler.
![]() GO FIGURE
GO FIGURE
Why is it reasonable to take account of only the large lobes of the Be hybrid orbitals in considering the bonding to F?
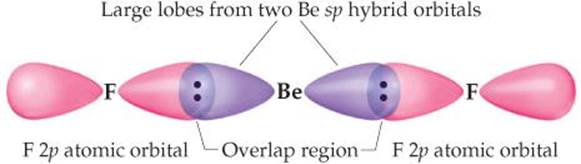
![]() FIGURE 9.16 Formation of two equivalent Be—F bonds in BeF2.
FIGURE 9.16 Formation of two equivalent Be—F bonds in BeF2.
![]() GIVE IT SOME THOUGHT
GIVE IT SOME THOUGHT
What is the orientation of the two unhybridized p orbitals on Be with respect to the two Be—F bonds?
sp2 and sp3 Hybrid Orbitals
Whenever we mix a certain number of atomic orbitals, we get the same number of hybrid orbitals. Each hybrid orbital is equivalent to the others but points in a different direction. Thus, mixing one 2s and one 2p atomic orbital yields two equivalent sp hybrid orbitals that point in opposite directions (Figure 9.15). Other combinations of atomic orbitals can be hybridized to obtain different geometries. In BF3, for example, mixing the 2s and two of the 2p atomic orbitals yields three equivalent sp2 (pronounced “s-p- two”) hybrid orbitals (![]() FIGURE 9.17).
FIGURE 9.17).
The three sp2 hybrid orbitals lie in the same plane, 120° apart from one another. They are used to make three equivalent bonds with the three fluorine atoms, leading to the trigonal-planar molecular geometry of BF3. Notice that an unfilled 2p atomic orbital remains unhybridized. This unhybridized orbital will be important when we discuss double bonds in Section 9.6.
![]() GIVE IT SOME THOUGHT
GIVE IT SOME THOUGHT
In an sp2 hybridized atom, what is the orientation of the unhybridized p atomic orbital relative to the three sp2 hybrid orbitals?
An s atomic orbital can also mix with all three p atomic orbitals in the same sub-shell. For example, the carbon atom in CH4 forms four equivalent bonds with the four
![]() GO FIGURE.
GO FIGURE.
How many atomic orbitals contribute to form the three sp2 hybrid orbitals?
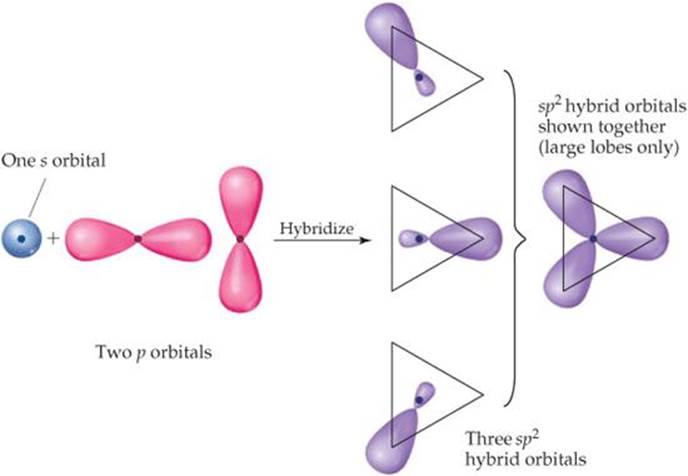
![]() FIGURE 9.17 Formation of sp2 hybrid orbitals.
FIGURE 9.17 Formation of sp2 hybrid orbitals.
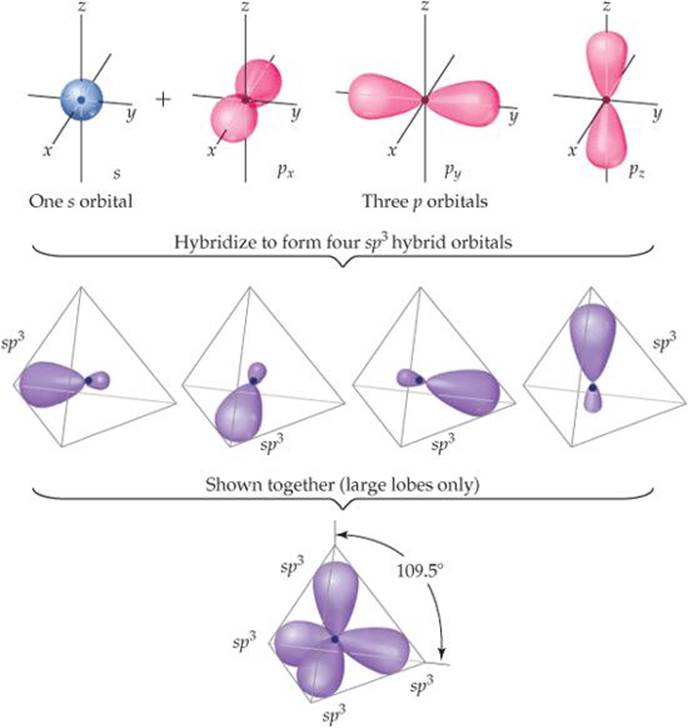
![]() FIGURE 9.18 Formation of sp3 hybrid orbitals.
FIGURE 9.18 Formation of sp3 hybrid orbitals.
hydrogen atoms. We envision this process as resulting from the mixing of the 2s and all three 2p atomic orbitals of carbon to create four equivalent sp3 (pronounced “s-p- three”) hybrid orbitals. Each sp3 hybrid orbital has a large lobe that points toward one vertex of a tetrahedron (![]() FIGURE 9.18). These hybrid orbitals can be used to form two-electron bonds by overlap with the atomic orbitals of another atom, such as H. Using valence-bond theory, we can describe the bonding in CH4 as the overlap of four equivalent sp3 hybrid orbitals on C with the 1s orbitals of the four H atoms to form four equivalent bonds.
FIGURE 9.18). These hybrid orbitals can be used to form two-electron bonds by overlap with the atomic orbitals of another atom, such as H. Using valence-bond theory, we can describe the bonding in CH4 as the overlap of four equivalent sp3 hybrid orbitals on C with the 1s orbitals of the four H atoms to form four equivalent bonds.
The idea of hybridization is also used to describe the bonding in molecules containing nonbonding pairs of electrons. In H2O, for example, the electron-domain geometry around the central O atom is approximately tetrahedral (![]() FIGURE 9.19). Thus, the four electron pairs can be envisioned as occupying sp3 hybrid orbitals. Two of the hybrid orbitals contain nonbonding pairs of electrons, and the other two form bonds with the hydrogen atoms.
FIGURE 9.19). Thus, the four electron pairs can be envisioned as occupying sp3 hybrid orbitals. Two of the hybrid orbitals contain nonbonding pairs of electrons, and the other two form bonds with the hydrogen atoms.
So far our discussion of hybridization has extended only to period 2 elements, specifically carbon, nitrogen, and oxygen. The elements of period 3 and beyond introduce a new consideration because in many of their compounds these elements have more than an octet of electrons in the valence shell, as we saw in Section 9.2. How do we analyze the bonding in compounds such as PCl5, SF6, or BrF5? The use of only s and p orbitals on the central atom limits us to four hybrid orbitals, yet in these compounds the central atom is involved in bonding to five or six other atoms.
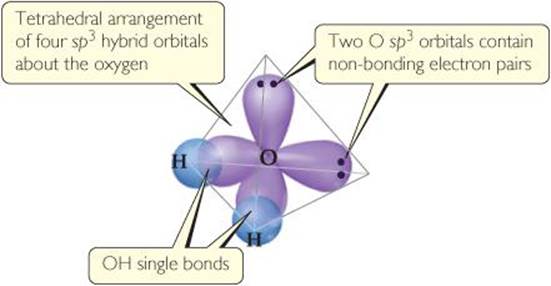
![]() FIGURE 9.19 Hybrid orbital description of H2O.
FIGURE 9.19 Hybrid orbital description of H2O.
For such elements, the number of hybrid orbitals formed could be increased by including valence-shell d orbitals. For example, to explain the bonding in SF6 we could include two sulfur 3d orbitals in addition to the 3s and three 3p orbitals. These six atomic orbitals could make six hybrid orbitals, but there is more involved in hybridization than simply finding a set of orbitals that point in the right directions; we must also consider orbital energies. The sulfur 3d orbitals lie substantially higher in energy than the 3s and 3p orbitals. The amount of energy needed to form the six hybrid orbitals is greater than the amount returned by forming bonds with the six fluorine atoms. Theoretical calculations seem to show that the sulfur 3d orbitals do not participate to a significant degree in the bonding between sulfur and the six fluorine atoms.
The valence-bond model we have developed for period 2 elements works well for compounds of period 3 elements so long as we have no more than an octet of electrons in the valence-shell orbitals. Thus, for example, it is appropriate to discuss the bonding in PF3 or H2Se in terms of hybrid s and p orbitals on the central atom. However, the model turns out not to be appropriate when there is more than an octet of electrons about the central atom. How then do we account for the bonding in SF6 and other compounds of the main group elements in which the central atom has more than an octet of valence electrons? To address that question from the viewpoint of bonding theory requires a treatment beyond the scope of a general chemistry text. Fortunately, the VSEPR model, although it does not explain the bonding in such molecules, can accurately predict their geometries.
This discussion points up the important fact that models in science are not reality but rather are our attempts to describe aspects of reality that we have been able to measure, such as bond distances, bond energies, molecular geometries, and so on. A model may work well up to a certain point but not beyond it, as with the idea of hybrid orbitals. The hybrid orbital model for period 2 elements has proven very useful and is an essential part of any modern discussion of bonding and molecular geometry in organic chemistry. When it comes to substances such as SF6, however, we encounter the limitations of the model.
Hybrid Orbital Summary
Overall, hybrid orbitals provide a convenient model for using valence-bond theory to describe covalent bonds in molecules in which the molecular geometry conforms to the electron-domain geometry predicted by the VSEPR model. The picture of hybrid orbitals has limited predictive value. When we know the electron-domain geometry, however, we can employ hybridization to describe the atomic orbitals used by the central atom in bonding.
The following steps allow us to describe the hybrid orbitals used by an atom in bonding:
1. Draw the Lewis structure for the molecule or ion.
2. Use the VSEPR model to determine the electron-domain geometry around the central atom.
3. Specify the hybrid orbitals needed to accommodate the electron pairs based on their geometric arrangement (![]() TABLE 9.4).
TABLE 9.4).
These steps are illustrated in ![]() FIGURE 9.20, which shows how the hybridization at N in NH3 is determined.
FIGURE 9.20, which shows how the hybridization at N in NH3 is determined.
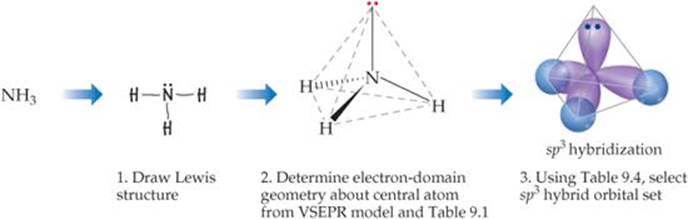
![]() FIGURE 9.20 Hybrid orbital description of bonding in NH3. Note the comparison with Figure 9.6. Here we focus on the hybrid orbitals used to make bonds and hold nonbonding electron pairs.
FIGURE 9.20 Hybrid orbital description of bonding in NH3. Note the comparison with Figure 9.6. Here we focus on the hybrid orbitals used to make bonds and hold nonbonding electron pairs.
TABLE 9.4 • Geometric Arrangements Characteristic of Hybrid Orbital Sets
SAMPLE EXERCISE 9.5 Hybridization
Indicate the orbital hybridization around the central atom in NH2–.
SOLUTION
Analyze We are given the chemical formula for a polyatomic anion and asked to describe the type of hybrid orbitals surrounding the central atom.
Plan To determine the central atom hybrid orbitals, we must know the electron-domain geometry around the atom. Thus, we draw the Lewis structure to determine the number of electron domains around the central atom. The hybridization conforms to the number and geometry of electron domains around the central atom as predicted by the VSEPR model.
Solve The Lewis structure is
![]()
Because there are four electron domains around N, the electron-domain geometry is tetrahe-dral. The hybridization that gives a tetrahedral electron-domain geometry is sp3 (Table 9.4). Two of the sp3 hybrid orbitals contain nonbonding pairs of electrons, and the other two are used to make bonds with the hydrogen atoms.
PRACTICE EXERCISE
Predict the electron-domain geometry and hybridization of the central atom in SO32–.
Answer: tetrahedral, sp3