CHEMICAL BIOLOGY
Calcium Signaling: Encoding and Decoding
Marisa Brini and Laura Fedrizzi, Department of Biochemistry and Department of Experimental Veterinary Sciences, University of Padova, Padova, Italy
Ernesto Carafoli, Venetian Institute of Molecular Medicine (VIMM), Padova, Italy
doi: 10.1002/9780470048672.wecb048
Calcium is a universal second messenger that controls many cellular reactions. Considering its pleiotropic role, it seems evident that the cells, to get a specific message in the proper time and manner, need precise and efficient mechanisms to encode and decode Ca2+ signals. Generally, extracellular stimuli are converted in a transient increase in cytosolic Ca2+ concentration, [Ca2+]c, which, in turn, modulates cell function. In the last two decades, improvements in the development of probes and instrumentation for Ca2+ imaging have led to the discovery that the coordinated action of different players is responsible for a complex spatio-temporal organization of the Ca2+ signal. It is intriguing to observe that cells can encode and discriminate Ca2+ signals not only according to their magnitude but also according to their localization (microdomains) and shape;i.e., cells can discriminate between sustained and oscillatory signals. Even more, in the case of oscillations, messages can be read differently according to the frequency of the oscillatory signals. The mechanisms by which cells decode Ca2+ signals are now explored in numerous laboratories. This article focuses on the autoregulation properties of the Ca2+ signals. It will show that Ca2+ itself is central in the regulation of the Ca2+ signal. It will also show that it can act as a first and second messenger and that it can modulate the activity and the availability of the other players in the signaling operation.
Eukaryotic cells are surrounded by media containing free Ca2+ concentrations that exceed 1 mM, but they manage to maintain a free Ca2+ concentration in the cytoplasm that is four orders of magnitude lower. The very low internal concentration is maintained by the active transport of Ca2+ ions against their concentration gradient by Ca2+ pumps in the plasma membrane, in the endoplasmic/sarcoplasmic reticulum (ER/SR), and in the Golgi membranes. The plasmalemmal Na+/Ca2+ exchangers also play a role, particularly in heart and skeletal muscle. The cells invest energy in this process that not only preserves the low [Ca2+]c but also generates an intracellular source of Ca2+ within the lumen of intracellular organelles, essentially, the ER/SR and the Golgi apparatus. Generally, extracellular stimuli are converted in a transient increase in the [Ca2+]c, which, in turn, activates cellular functions. The sources of Ca2+ are both extracellular and intracellular; i.e., Ca2+channels in the plasma membrane and in the intracellular membranes are critical in the control of cellular Ca2+ homeostasis. To guarantee the specificity of the signal transmission, the cell organizes dynamically the Ca2+ fluctuations in the cytosol by varying the distribution, the type, and the availability of the different Ca2+ transporters, and it increases the spatial and temporal complexity of Ca2+homeostasis by compartmentalizing the signals into the organelles. The ER was originally considered to be the sole dynamic Ca2+ regulator in the cell, but it has now become clear that the nucleus, the mitochondria, the Golgi apparatus, the endosomes/lysosomes, and the secretory vesicles also play fundamental roles (Fig. 1). Recent methodological developments have revealed that, in living cells, these systems are strictly interconnected. The control of their Ca2+ homeostasis is not only essential in the control of organelle-specific processes, but it is also fundamental in the overall dynamic modulation of the Ca2+ signaling in the cytosol. Many protein components of this signaling cascade have been cloned and characterized: Strikingly, many exist as different isoforms, the number of which is further increased through mechanisms of alternative splicing of the primary transcripts. The reason for this redundancy is not yet completely understood, but emerging evidence suggests that each variant of the Ca2+-controlling proteins has a precise role in the shaping of the Ca2+ signal. Importantly, it has also emerged that Ca2+ may even regulate the generation and the transmission of its signal by controlling the expression of its own transporters.
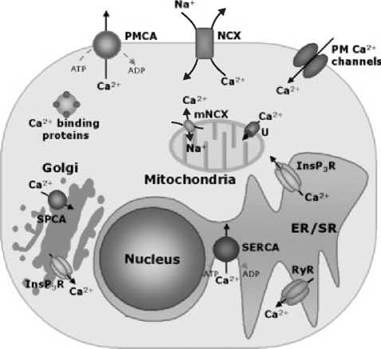
Figure 1. A schematic representation of the Ca2+ transporters of animal cells. Plasma membrane (PM) channels are gated by potential, ligands or by the emptying of Ca2+ stores. Channels in the ER/SR are opened by InsP3 or cADPr (the cADPr channel is sensitive to ryanodine and is thus called RyR). ATPase pumps are found in the PM (PMCA), in the ER/SR (SERCA), and in the Golgi apparatus (SPCA). The nuclear envelope, which is an extension of ER, contains the same transporters of the latter. NCXs are located in the PM (NCX) and in the inner mitochondria membrane (mNCX). A uniporter (U) driven by the internal negative potential (-180 mV) transports Ca2+ into mitochondria. Ca2+-binding proteins are represented with a sphere containing the four EF-hands Ca2+-binding sites.
Sources and Types of Ca2+ Signaling
Plasma Membrane Ca2+ Entry Mechanisms
Several plasma membrane channels control Ca2+ entry from the external medium. The gating of these channels may depend on membrane potential [in the so-called voltage-operated Ca2+channels (VOCCs) (1)], or on ligand binding [in the case of receptor-operated Ca2+ channels (ROCCs) (2)], or on a still poorly understood mechanism linked to the emptying of intracellular Ca2+ stores. VOCCs are found not only in excitable cells such as neurons, skeletal muscle, and heart, but also in nonexcitable cells. Six classes (termed L, N, P, T, R, and Q) have been identified based on physiologic and pharmacologic properties. Structurally, all VOCCs are complexes of five subunits, termed α1, α2, δ, β, and γ, that assemble into large macromolecular complexes and that are encoded by different genes. α1, the largest subunit, contains the conduction pore, the voltage sensor, the gating apparatus, and the sites of channel regulation, e.g., by protein kinases, by drugs, and by toxins. Much of the diversity of Ca2+ channel types originates from the variety of α1 subunits, and at least 10 different genes encoding for different α1 subunits have so far been identified in mammals. ROCCs are activated by the interaction of ligands with plasma membrane receptors. L-glutamate is the most abundant excitatory transmitter in the vertebrate central nervous system. It activates two general classes of receptors, the “ionotropic” receptors, which are ionic channels, and the “metabotropic” receptors, which are coupled to G-proteins, which activate phospholipase C (PLC) and promote intracellular responses. Three forms of ionotropic receptors have been characterized and named after their most widely used agonist: the kainate (KA), the α-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA), and the N-methyl D-aspartate (NMDA) receptors. AMPA and NMDA receptors colocalize at the same postsynaptic membrane, and their close functional interdependence has important roles in the processes of memory storage and learning. A heterogeneous group of channels (most of which belong to the so-called transient receptor potential family, TRPs) are activated by a variety of factors, including mechanical stretch, osmolarity, temperature, second messengers, G-proteins, protein-protein interactions, and so on (3). The molecular nature of an additional group of channels, in this case activated by arachidonic acid, is still undetermined (4). Whatever the nature and gating properties of a channel, it is easily envisaged that upon its opening, a high concentration of Ca2+ will be generated at its mouth, on the inner side of the plasma membrane. In general terms, the peak amplitude and spatial diffusion of the Ca2+ microdomain formed at the internal mouth of a Ca2+ channel and its immediate neighborhood will depend on the following: the conductance of the channel itself, its Ca2+ selectivity, the concentration of Ca2+ in the extracellular medium, the membrane potential, and the nature and amount of the intracellular Ca2+ buffers.
Intracellular Ca2+ Release Mechanisms
The release of Ca2+ from internal stores, usually the ER or its muscle equivalent, the SR, is controlled by Ca2+ itself, or by an expanding group of messengers, such as inositol 1,4,5 tris-phosphate (InsP3), cyclic ADP ribose (cADPR), nicotinic acid adenine dinucleotide phosphate (NAADP), and sphingosine-1-phosphate (S1P), that either stimulate or modulate the release channels on the internal stores. The Ca2+ release channels represent the molecular component of the Ca2+ handling machinery of the intracellular stores. They belong to two families, the so-called InsP3 receptors (InsP3Rs) and Ryanodine receptors (RyRs) (5). The heterogeneous distribution of these channels within intracellular membranes is one of the key factors in determining the spatial heterogeneity of the Ca2+ signals and thus the formation of Ca2+ microdomains. The two channel types have been conserved during evolution, as a significant degree of homology characterizes the sequences of the domains next to the C -terminus that span the membrane and contribute to the assembly of the channel proper. Both are tetramers composed of identical subunits, but the mechanism of their activation is different.
In cardiac and skeletal muscle, a highly structured morphological architecture allows the generation of Ca2+ microdomains at the surface of the SR; these microdomains are a key component of the trigger for firing the Ca2+ signals necessary for cell activation according to the mechanism known as Ca2+-induced-Ca2+-release (CICR) (6). Indeed, in both cell types, the physiologic stimulatory signal leading to contraction is conveyed by action potentials: A plasma membrane depolarization travels via the opening of voltage-dependent Na+ channels and reaches the cell interior through invaginations (the T tubules) in which VOCCs are located; this process causes the influx of Ca2+ that is per se insufficient to induce the physiologic response (the sliding of the acto-myosinic contractile apparatus), but it represents the trigger for the release of Ca2+ (particularly in the heart) from the large intracellular Ca2+ reservoir (the SR), through the RyRs (7). In skeletal muscle, direct coupling between the two molecules (VOCCs and RyRs) is believed to cause the opening of RyRs; thus, the influx of Ca2+ through VOCCs plays a facilitatory, but not a necessary, role. The interaction between the two channels is thought to be the necessary event to cause activation of the RyR isoform of skeletal muscle (RyR1), and then Ca2+ release through the RyR1 is amplified by CICR. In heart, no direct physical interaction occurs between the two types of channels, and thus a high [Ca2+] in the proximity of the RyR2 (the cardiac RyR isoform) represents the essential activatory signal (8). Therefore, in heart, Ca2+ influx through VOCCs is necessary to trigger SR Ca2+ release through the RyR2 via the process of CICR.
In this context, the Ca2+ sensitivity of the RyRs, as well as the evaluation of the local Ca2+ concentration to which they are exposed, is a key factor in determining the excitability of the muscle fiber and the efficiency and duration of contraction. As a result, a great deal of work has concentrated on identifying the “fundamental” Ca2+ signaling. Rapid Ca2+ imaging of both cardiac and skeletal muscle has revealed the occurrence of transient, local increases in Ca2+ concentration, denominated Ca2+ sparks (9) that were attributed to the opening of single RyR channels, or more likely, a cluster of RyRs (10, 11).
The occurrence of elementary signals, generated by the opening of a spatially restricted group of channels (12) and denominated “Ca2+ puffs” (13), is also shared by the InsP3-dependent signaling system. Ca2+ puffs denote the “Ca2+ excitability” of the cell, but the induction of a physiologic response requires the coalescence of these elementary events into a larger rise, which may be limited to a portion of a polarized cell or may diffuse to the whole cell body in a truly global Ca2+ signal. The transition from elementary to global events, and its regulatory mechanisms, is thus critical in determining the final cellular outcome of the InsP3-dependent Ca2+ signal. Numerous stimuli function through the activation of PLC to generate InsP3. Several isozymes of PLC have been described: all of them specific for phosphatidyl-inositol (PtdIns, PI), a phospholipid that is predominantly present in the inner leaflet of the plasma membrane, and its mono (PtdIns-4-P, PIPi) and bis-phosphorylated forms (PtdIns-4,5-P2, PIP2). Diacylglycerol (DAG) and phosphorylated inositols are formed, with 1,4,5 InsP3 being the only one among the numerous inositol phosphate isomers that is capable of releasing Ca2+ from intracellular stores by interacting with a specific receptor. Three genes encode the InsP3Rs. They are ubiquitously expressed; type 1 is expressed in particularly high levels in Purkinje neurons in the cerebellum. The differential contribution of InsP3R isoforms to Ca2+ signaling is now being explored. Studies on the DT40 chicken lymphoma cell line, in which one or more of the InsP3R genes were systematically eliminated by homologous recombination, have shown that the expression of isoform 2 is necessary for the generation of Ca2+ oscillations after cell stimulation (14).
The InsP3R is operated by InsP3, but it is also modulated by Ca2+. Low lumenal Ca2+ has been proposed to stimulate the opening of the Ca2+ channel, but the matter is still being debated. The effects of cytosolic Ca2+ on the receptor are better documented. Even if they may vary, depending on concentration, cell type, or experimental conditions, the effects of Ca2+ are biphasic. They are stimulatory at <300 nM and inhibitory at >300 nM (15, 16).
Intracellular Ca2+-Removing Mechanisms
During the generation of a Ca2+ transient, the “on” reactions are counteracted by “off” reactions, during which time various pumps and exchangers remove Ca2+ from the cytoplasm. These mechanisms are essential in maintaining the resting level of Ca2+ at approximately 100 nM and in ensuring the Ca2+ loading of internal stores. As mentioned, three different molecular components are involved in the “off’ reactions: the plasma membrane Ca2+ -ATPase (PMCA), the Na+/Ca2+ exchanger (NCX), and the sarco/endoplasmic Ca2+-ATPase (SERCA). PMCAs represent the main Ca2+ -extrusion system of all eukaryotic cells and have high Ca2+ affinity. In mammals, four separate genes encode distinct PMCA isoforms. PMCA1 and PMCA4 are ubiquitously expressed, whereas PMCA2 and PMCA3 are expressed almost exclusively in the central nervous system. Complex patterns of alternative RNA splicing generate additional isoform variability: Of interest are variants in which the alternative splicing occurs at site C, located in the C-terminal tail of the protein, which involves the region containing the calmodulin-binding domain (see below). The calmodulin (CaM)-binding domain, in the absence of CaM, interacts with two sites next to the active site of the PMCA maintaining the pump in an inhibited state. CaM removes its binding domain from its intramolecular receptors, freeing the pump from autoinhibition. C site splicing occurs in all isoforms and, in general, causes the inclusion of one (or two) additional exons, inducing a premature truncation of the protein, which now has a shorter regulatory domain (termed CII or a variant) that differs significantly from that of the variant termed CI or b (17).
The other plasma membrane Ca2+-exporting system, the NCX, is particularly active in excitable cells, e.g., heart, which periodically experiences the need to rapidly eject large amounts of calcium. It has low Ca2+ affinity but high Ca2+-transporting capacity. Three basic exchanger gene products are known, NCX1, NCX2, and NCX3. The first two products are ubiquitously distributed in tissues, whereas NCX3 is restricted to the brain (18). The SERCA pumps are encoded by three genes and are differently distributed in the animal tissues. SERCA1 is largely expressed in fast-twitch skeletal muscle. SERCA2 has two splicing variants: isoform 2b, which is ubiquitously distributed, and isoform 2a, which is dominantly expressed in cardiac muscle. SERCA3 is instead expressed in nonmuscle cells. Recently, the tertiary structure of the SERCA1a pump has been solved, and it has confirmed the predicted architecture containing 10 transmembrane domains (19).
The diverse PMCA, SERCA, and NCX isoforms are expressed in a tissue- and development-specific manner, and their apparent redundancy probably enables the cells to select the combination of reactions that exactly meet their specific Ca2+-signaling requirements.
Functional Significance of the Different Modes of Ca2+ Signal Transmission
Different cell types use distinct Ca2+ signals, as suits best their physiology. In particular, the possibility of local and global Ca2+ signaling permits the control of separate processes in the same cell.
Emerging evidence reveals that cells use local Ca2+ signals, i.e., spatially confined high Ca2+ concentration microdomains, to obtain specific and rapid effects such as the release of the contents of synaptic or secretory vesicles, the activation of ion channels, mitochondrial energy metabolism, and the generation of nuclear specific Ca2+ signals (20, 21). Confined Ca2+ signals at the internal mouth of the channels may thus remain localized and transmit the signal to targets in the immediate vicinity or trigger a chain of autocatalytic Ca2+-releasing events that results in the generation and spreading of Ca2+ waves across the cell. Ca2+ waves have been classified based on the speed of their motion (22). Global Ca2+ signals, such as waves and oscillations, are preferentially used when the targets to reach are distributed throughout the cell. Many processes require prolonged stimulation to be activated, such as fertilization, axonal growth of cortical neurons, neuronal cell migration, exocytosis, and gene transcription. Ca2+ waves can also spread from one cell to the next, thus coordinating the activity of groups of cells within a tissue. For example, in perfused intact liver, hormones linked to the formation of InsP3 evoke Ca2+ transients with an oscillatory pattern, the frequency of which depends on the concentration of the agonist. The spikes spread as wave through the cytoplasm, the nucleus, and also through gap junctions to the entire liver lobule to coordinate the metabolic liver function (23).
Information is also encoded within the frequency of Ca2+ oscillations that occur in the cytosol. Oscillations can derive either from fluctuations of the entry of external calcium or of the release from internal stores. The former occur primarily in excitable cells, after the periodic opening of plasma membrane Ca2+ channels, such as induced, for example, by the rhythmic changes of the plasma membrane potential of the heart or by bursts of action potential in neurons. In nonexcitable cells, the predominant mechanism of [Ca2+]c elevation is from the activation of plasma membrane receptors coupled to G-proteins and to the phospho-inositide pathway. In these cells, the oscillatory pattern of Ca2+ increases is not dependent on the periodic opening and closing of plasma membrane Ca2+channels, but on cycles of Ca2+ release and uptake from the intracellular compartment sensitive to InsP3. Recent works suggest at least two possible mechanisms for the generation of oscillatory Ca2+signals: either an oscillatory production of InsP3 or an oscillatory inactivation of InsP3Rs. The two mechanisms appear to operate differentially according to the cell type. The fluctuations in InsP3 may be controlled by Ca2+ itself through the regulation of membrane PLC that generates InsP3 from PIP2, or through regulatory proteins, which act directly on G-proteins, thus affecting the downstream InsP3production. Figure 2 summarizes the different shapes of Ca2+ signals and some of their biologic targets.

Figure 2. Scheme of the main cellular events modulated by local (microdomains) and global (waves and oscillations) Ca2+ signaling.
How are the Ca2+ Messages Decoded?
The ability to use Ca2+ in different modes helps cells to achieve a multitude of signals varying in amplitude, frequency, kinetics, and localization, as well as to avoid the deleterious effects associated with sustained Ca2+ increases. Cells avoid death either by using low amplitude Ca2+ signals or, more usually, by delivering the signals as brief transients. It is intriguing to understand how the encoded message could regulate such a large number of different processes. The explanation probably lies in the fact that different “forgers” personalize the message.
After cell stimulation, Ca2+ flows into the cells and interacts with different Ca2+-binding proteins that function either as Ca2+ effectors (i.e., decoding the message) or Ca2+ buffers. Proteins able to bind Ca2+ with the affinity and specificity required for the regulation of its concentration in the intracellular environment generally belong to the so-called EF-hand family (24). The family contains hundreds of members, of which the most intensively studied is the ubiquitous CaM. CaM (and a number of homologous proteins) consists of two independently folded domains connected by a flexible long helical segment. Each domain contains one pair of EF hands. Upon Ca2+ binding, structural rearrangements occur and CaM collapses into a hairpin structure around the binding domains of target proteins. Clearly, the decoding of the Ca2+ signal by EF-hand proteins is a sophisticated operation, which is not restricted to the relatively simple process of reversibly binding Ca2+ for buffering purposes. In fact, the buffers function to fine-tune the spatial and temporal properties of Ca2+ signals. They can alter both the amplitude and the recovery time of individual Ca2+ transients. These buffers have different properties and expression patterns. For example, calbindin-D28 (CB) and calretinin (CR) are fast buffers, whereas parvalbumin (PV) has much slower binding kinetics and high affinity for Ca2+. The physiologic roles of CB, CR, and PV have been particularly studied in neurons. These studies have been facilitated by the recent generation of mouse strains deficient in these proteins. CR is principally expressed in cerebellar granule cells and their parallel fibres, whereas PV and CB are present throughout the axon, soma, dendrites, and spines of Purkinje cells. PV is additionally found in a subpopulation of inhibitory interneurons, the stellate, and basket cells. Studies on deficient mice, together with in vitro work and the discovery of their unique cell type-specific distribution in the cerebellum suggest that these calcium-binding proteins have evolved as functionally distinct, physiologically relevant modulators of intracellular calcium transients. Analysis of different brain regions suggests that these proteins are involved in regulating calcium pools critical for synaptic plasticity (25). Interestingly, and unexpectedly, PV-/- fast-twitch muscles are considerably more resistant to fatigue than the wild-type controls. This effect was attributed mainly to the increased fractional volume of mitochondria in PV-/- fast-twitch muscle, where the mitochondria are suggested to functionally replace the slow-onset buffer PV based on similar kinetic properties of Ca2+ removal (26).
In addition to limiting the diffusion and the magnitude of Ca2+ transients by activating the Ca2+ extrusion systems and by buffering them using cytosolic Ca2+ buffers, it recently became evident that cells also use the spatial distribution of intracellular organelles, such as mitochondria (for a comprehensive review, see Reference (27)). The control of the ion diffusion throughout the cell has been found to control the shaping of cytosolic Ca2+ signals in different cell types. In pancreatic acinar cells, mitochondria strategically located beneath the granular region prevent the spreading of a Ca2+wave from the secretory pole toward the basolateral region by accumulating Ca2+; a similar role was reported for mitochondria in rat cortical astrocytes. In addition, the clearing of local [Ca2+]c in the proximity of Ca2+ release channels plays a role in the modulation of their activity. The first example of this effect was reported in Xenopus oocytes where mitochondria buffer microdomains of [Ca2+]cregulating the open probability of InsP3 channels, relieving the inhibitory effect of Ca2+. As a consequence, the rate of Ca2+ efflux from ER and, in turn, the shape of cytoplasmic Ca2+ waves, becomes modulated. A similar role for mitochondria has been described also in mammalian cells where mitochondria suppress [Ca2+] positive (or negative) effects on the InsP3 or on ryanodine channels or plasma membrane channels. By dissipating these local [Ca2+] microdomains, mitochondria buffer the Ca2+ that enters T cells via store-operated Ca2+ channels: They sequester Ca2+ during periods of rapid Ca2+ entry and release it slowly after Ca2+ entry has ceased. The idea that mitochondria can prolong the period of [Ca2+]c elevation in response to a transient episode of Ca2+ influx by slowly releasing stored Ca2+ was already proposed as important for Ca2+-dependent processes such as exocytosis and synaptic transmission.
We still have little understanding of how cells actually decode the information contained in the different “shapes” of the Ca2+ signaling and, in particular, in the frequency of Ca2+ signals. The molecular machines that are responsible for decoding frequency-modulated Ca2+ signals include CaM kinase II (CaMKII) and protein kinase C (PKC). CaMKII is a multimer, consisting of 6-12 monomers that could be identical or different. It has a broad tissue distribution, being particularly abundant in the forebrain, where it is supposed to be particularly important in decoding the frequencies of synaptic inputs. CaMKII phosphorylates and regulates multiple cellular targets that contribute to neurotransmission, neuronal plasticity, cell excitability, gene expression, secretion and cell shape, and, especially, memory formation and storage (28). Evidence that CaMKII could act as frequency decoder of Ca2+ oscillations comes from the work of De Koninck and Schulman (29), who had shown that, after the threshold for kinase activation was reached and some subunits of the enzyme had become autophosphorylated, the response of the kinase to low frequency stimuli increased, presumably because CaMKII autophosphorylation is functionally cooperative.
PKC is a member of a family of Ser/Thr phosphotransferase that is involved in numerous cellular signaling pathways. These enzymes possess two regulatory domains, C1 and C2, that are the targets of different second messengers. Conventional and novel PKCs migrate to the plasma membrane in response to increased levels of DAG, resulting in complete activation of the isoenzymes. Activation is produced by the direct binding of DAG to a motif known as protein kinase C homology-1 (C1) domain. The main role of C2 domains in conventional PKCs is to act as the Ca2+-activated membrane-targeting domain. In the absence of receptor-mediated lipid-hydrolysis, PKC isoenzymes are, in most cases, cytosolic and autoinhibited by the binding of a pseudo substrate motif to the substrate-binding site. Receptor-mediated generation of DAG and elevation in [Ca2+]c result in the membrane recruitment of conventional PKC molecules. Once fully membrane-associated, the pseudo substrate motif is released, allowing substrate binding and phosphorylation. The use of the green fluorescent protein (GFP) has shown that conventional PKC isoenzymes undergo oscillatory plasma membrane associations, which are “phase-locked” with the underlying receptor-mediated oscillations in [Ca2+]c. Each oscillatory membrane association may lead to a burst of PKC activity, which in turn induces transient bursts in substrate phosphorylation. As Ca2+ returns to the basal level between oscillations, PKC regains the autoinhibited conformation. This pause in PKC activity allows the dephosphorylation of the substrate to dominate (30, 31). Such precise control of PKC activity is crucial to decoding the information contained in [Ca2+]c oscillations, and it is worthwhile to note that the other Ca2+oscillation decoder (CaMKII) does not fully deactivate between oscillations (29).
Recently, it has been proposed that Ca2+ signals could be decoded also through a pathway involving the Ras GTPases (32). In neuronal cells, Ca2+ microdomains generated through Ca2+ influx through the NMDA-Rs can efficiently activate the Ras/ERK cascade. The proposed mechanism for Ca2+ activation of Ras signaling is based on a spatially restricted localization of two enzymes (GAPs proteins), the activity of which modulates the conversion between Ras-GTP and Ras-GDP in a Ca2+-dependent manner. This confined coupling is essential in the physiology of neuronal cells, because the Ras/ERK pathway is important in eliciting two forms of synaptic plasticity, long-term depression (LTD) and long-term potentiation (LTP), both processes that have been proposed to form part of the cellular basis of learning and memory. AMPA-Rs trafficking in the postsynaptic membrane, a process that also controls LTP and LTD, appears to be modulated through the Ras/ERK pathway activation by CaMKII-mediated phosphorylation (33). Efficient Ca2+-mediated activation of the Ras/ERK cascade appears to be optimized according to the frequency of the Ca2+ oscillations thanks to the action of two Ca2+-regulated Ras GAPs, CAPRI and RASAL. These two proteins have been recently identified as sensors of distinct temporal aspects of the Ca2+ signals. Whereas CAPRI detects the intensity of the Ca2+ signal and undergoes transient association with the plasma membrane, RASAL senses the frequency by undergoing synchronous and repetitive oscillatory associations with the plasma membrane (34).
Calcium Controls the Expression of its Own Transporters
Control of gene expression by Ca2+ was identified in 1985 when it was shown that prolactin gene transcription was stimulated up to 200-fold by the elevation of intracellular Ca2+ in cultured CH3 cells (35). After this observation, the transcription of numerous other genes was also found to be stimulated by Ca2+. According to general consensus, Ca2+ acts through three major pathways (Fig. 3). The first pathway, which is probably the principal one, involves changes in the activity of several Ca2+ -dependent kinases and phosphatases, which in turn change the trans-activating properties of several transcription factors. The best-known proteins involved in this pathway are probably CaM-dependent kinases CaMKIV, CaMKII (36), and the CaM-dependent protein phosphatase calcineurin (37). All of these proteins need CaM to process the Ca2+ signal: They stimulate gene transcription by regulating the phosphorylation state of the transcription factor CREB, and, as a result, the extent of CRE (cAMP Response Element)-dependent transcriptional activation, or by dephosphorylating the transcription factor NF-AT, thus promoting its translocation to the nucleus, respectively.

Figure 3. Signaling pathways that participate in Ca2+-regulated gene expression. Two important enzymes (calcineurin, CN, and calmodulin-dependent kinases, CaMK), which by regulating the phosphorylated/dephosphorylated state of the transcription factors CREB and NF-AT, translate the Ca2+ signal into nuclear specific information, are indicated. The transcriptional regulator DREAM, which is an EF-hand Ca2+-binding protein, and the basic-helix-loop-helix (bHLH) transcription factors, which bind calmodulin (CaM) or S-100 proteins, are also shown. NLS, nuclear localization signal. Ca2+ levels, through the indicated pathways, directly regulate the transcription of the genes for the membrane Ca2+ transporters PMCA, NCX, and InsP3R.
The regulation of synaptic plasticity in neuronal cells is an important consequence of the activation of this pathway of gene expression, after Ca2+ entry from the extracellular environment. An interesting finding that recently emerged was that Ca2+ itself could control the expression of its own transporters, suggesting that the cell can finely adjust the amount, the type, and the distribution of the Ca2+ transporters according to its specific demands. Ca2+ regulation of the plasma membrane Ca2+ pump isoforms at the transcriptional level was firstly reported by Guerini et al. (38) in cerebellar granule neurons. When neurons were cultured under condition of partial membrane depolarization, which is required for their long term in vitro survival, they underwent a chronic and modest increase of the resting free Ca2+ concentration, which promotes the up regulation of PMCA2, 3, and 1CII (1a) (that are typical of the adult brain), and the down regulation of PMCA4CII (4a) (which is absent from adult rat cerebellum). Calcineurin regulates the disappearance of PMCA4a but not the up regulation of the other PMCA isoforms (39).
Interestingly, a similar pattern of remodeling also occurs for the expression of the other Ca2+ extrusion system of the plasma membrane, the NCX, which has a high level of expression in brain. Three separate genes encode for the three different isoforms of NCX, and, as in the case of PMCA, a number of splice variants are generated at least at the transcriptional level. It has been shown that during the maturation of neurons, the number of splicing variants of NCX1 decreased but the total amount of NCX1 protein did not change significantly. At variance with this result, the amount of NCX2 protein increased dramatically with time if the cultured neurons were kept in low KCl-containing medium, reflecting the behavior of the isoform in the cerebellum during the first week, but became rapidly down regulated after partial depolarization of the plasma membrane (40). The NCX3 gene was also affected by the depolarizing treatment: Its transcription became up regulated when Ca2+ influx is promoted by high KCl treatment. Interestingly, the NCX2 transcript up regulation was dependent on the activation of calcineurin, whereas the effects on the NCX1 and NCX3 genes were instead calcineurin-independent. Further reports have indicated that the Ca2+-mediated increase in the expression of type 1 InsP3R in cerebellar granules (41) and in hippocampal neurons (42) is mediated by Ca2+ influx through L-type channels or NMDA-Rs. The expression of type 1 InsP3R appears to be regulated through the activity of calcineurin, which dephosphorylates the NF-AT transcription factor, promoting its translocation to the nucleus and transcription activation. These regulation pathways underline the importance of regulating cell Ca2+ with absolute precision: To promote the survival of the neurons, cytosolic Ca2+ must increase, but only to the relatively modest level that is necessary and no more. Evidently, to better control Ca2+ levels, cells adjust the abundance and the type of the different Ca2+ transporters.
Another important recent development in the transcriptional autoregulation of the Ca2+message is that linked to the second pathway that Ca2+ uses to control gene expression: Ca2+ ions bind directly to the transcriptional regulator DREAM (DRE Antagonist Modulator) and change its affinity for the DNA, relieving the repression on the transcription of specific target genes (43). DREAM belongs to the family of neuronal calcium sensors. It contains 4 EF-hands and acts as a transcription silencer for a large number of genes by binding to specific DRE (Downstream Regulatory Element) sites in the 5' UTR region of the gene promoters. When Ca2+ becomes bound to DREAM, presumably as a result of its increase in the intracellular (intranuclear) environment, the DRE sites release DREAM and transcription resumes. The first discovered target gene for DREAM was that for the human prodynorphin, a protein involved in memory acquisition and pain (43); but an increasing number of genes have now been found to be regulated by this Ca2+-sensitive transcriptional repressor. Very recently, DREAM has been shown to control the transcription of the gene for NCX3, an isoform of NCX that is important in Ca2+ extrusion in neurons (44). Overexpression of a DREAM EF-mutant (EFm- DREAM) insensitive to Ca2+ in hippocampus and cerebellum of transgenic mice significantly reduced NCX3 mRNA and protein levels. Cerebellar granules from EFmDREAM transgenic mice displayed increased levels of cytosolic Ca2+, lost the ability to efficiently export Ca2+, and were more vulnerable to increased Ca2+ influx after partial opening of VOCCs. However, they survived better under conditions of reduced Ca2+ influx, suggesting that DREAM plays a role in the autoregulation of the Ca2+ signal in neurons.
Lastly, the third mode that Ca2+ uses to control gene expression must be mentioned, even if no evidence has yet conclusively shown that this mode is employed to control the transcription of the Ca2+ transporters. This pathway involves the basic-helix-loop-helix (bHLH) transcription factors that, after Ca2+-dependent interaction with CaM or with the S-100 proteins, modify the ability to bind to DNA and thus to activate transcription (45).
Measuring Ca2+ Concentration
Ca2+ probes (also known as indicators, reporters, or sensors) are molecules that form selective and reversible complexes with Ca2+ ions. The physicochemical characteristics of the Ca2+-free and Ca2+-bound form are sufficiently different to enable their relative concentrations to be measured. They can be divided in two main categories: synthetic Ca2+ indicators and protein-based Ca2+indicators. In the late 1970s, Roger Tsien synthesized the first fluorescent Ca2+ probe for intracellular use. Its structure was based on the selective Ca2+-chelator EGTA. The portion of the molecule that binds Ca2+ is a carboxylic backbone perfectly adapted to the dimension of the ion, which confers its specificity. A fluorophore group, associated with the carboxylic group, endows the molecule with fluorescent properties dependent on the binding of Ca2+ to the carboxylic cage. Tsien and co-workers modified the original molecule by esterification of the charged carboxylic groups, making it permeable to the plasma membrane of a living cell (46). In this form, the molecule cannot bind Ca2+; but once permeated through the plasma membrane, it is trapped in the cytoplasm thanks to the action of cellular esterases that hydrolyse the ester, yielding the active form of the indicator. Thanks to the simplicity of use, this type of indicators is enormously employed by many researches. Big developments have been made to improve their fluorescent signal that, together with the improvements of the instrumentation, have contributed to image at single cell level the changes in Ca2+concentration induced by physiologic stimuli.
In recent years, the wide diffusion of molecular biology techniques has extensively expanded the number of applications of protein probes in cell biology. Two types of protein probes currently employed derive from bioluminescent organisms. The first is the group of chemiluminescent proteins, which emit light usually in response to changes of a physiologic parameter, such as concentration of ATP or Ca2+. Among these proteins, the photoprotein aequorin (AEQ) has dominated the Ca2+-signaling field. The second group of protein probes is that of fluorescent proteins, of which GFP from Aequorea victoria is the “protagonist”.
AEQ was largely employed before the introduction of synthetic fluorescent probes, as it was extracted and purified from jellyfish and microinjected in giant cells to monitor Ca2+. A Ca2+-induced conformational change in the apoprotein leads to peroxidation of the coenzyme, which results in the release of blue light. The rate of the reaction depends on the Ca2+ concentration to which the photoprotein is exposed. The cloning of the AEQ cDNA in 1985 (47) opened the possibility to extend AEQ use to a large variety of cells by transfecting them with a plasmid that allows recombinant expression of exogenous protein. But the most important incentive to reconsider AEQ to monitor Ca2+ was the possibility to target it to a specific cell compartment by introducing in its sequence specific signal sequences. This approach has allowed the construction Ca2+ probes, which, in contrast to fluorescent dyes, are exclusively localized in the intracellular district of interest. The use of chimeric AEQs allowed substantial advances in our understanding of Ca2+ signaling, such as the interplay between the ER and mitochondria (48), the presence of subplasma membrane Ca2+ microdomains (49), and the role of Golgi apparatus as an important Ca2+ store (50). Despite its undoubted advantages, aequorin has a big defect: Although the amount of photons that are emitted from a cell population is more than adequate to measure Ca2+ concentration, the amount of photons that are emitted by a single cell is very low and not sufficient to guarantee a good space and time resolution. To overcome this deficiency, recombinant Ca2+ probes based on GFP were developed. Essentially, these indicators can be divided in two main groups based on their structure: double barrel probes, such as cameleons, made of two different coloured mutants of GFP connected by a Ca2+-sensitive linker and single barrel probes, such as camgaroos and pericams, based on a single GFP engineered to bear a Ca2+-dependent inserted sequence. Generally, CaM is used as a molecular switch, which changes its conformation on the binding of Ca2+. The first two GFP-based fluorescent Ca2+ indicators were developed in 1997 (51, 52). Both probes are based on a similar strategy: the change of Fluorescence Resonance Energy Transfer (FRET) between the two GFP mutants that is caused by the interaction between Ca2+-activated CaM and the targeted peptide. Although the cameleons were greatly improved over the original design, they still displayed insufficient signal-to-noise ratio when targeted to the organelles. To further increase the dynamic range of cameleons, the second generation probes (camgaroos and pericams) have been developed. In this construct, the GFP P barrel has been cut open and the original N- and C -termini linked together to create new termini located close to each other. The circular permutated (cp) GFP retains the ability to form a chromophore, but the permutation renders the chromophore more accessible to changes in the pH of the surrounding ambient. As protonation of residues surrounding the chromophore changes its ionization state, the fluorescence of all cpGFP is highly pH sensitive. This property has been exploited to detect Ca2+ by fusing CaM to cpGFP. In this way, the binding of Ca2+ mimics alkalinization or acidification, and so results in the increase or decrease of chromophore fluorescence. Further developments have been made to improve the Ca2+ sensors and, in particular, CaM has been substituted by troponin C (53) or by an artificial Ca2+-binding module to reduce the interaction with endogenous proteins (54), but the “perfect” Ca2+ probe is still missing.
Conclusion
More than 40 years of investigations of Ca2+ transport and Ca2+ actions have resulted in the well-defined concepts of intracellular Ca2+ homeostasis and Ca2+ signaling. The molecular mechanisms of Ca2+ transport between cells and extracellular space as well as within cells are clearly defined. We also know that Ca2+ signaling is extremely compartmentalized and that local and transient gradients could be responsible for spatial signal encoding. The study of the mechanisms of encoding/decoding the Ca2+ signal is fundamental to understanding the Ca2+-signaling specificity, and the enormous number of Ca2+-signaling studies carried out in cultured cells or in tissue slices has clearly contributed to the progression in this field. Now, it will be nice when the recent technological developments drive the analysis toward in vivo cellular imaging, where the effects of physiologic and pathologic stimuli could be investigated on the whole living organisms.
References
1. Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu. Rev. Cell Dev. Biol. 2000; 16:521-555.
2. Meldolesi J, Pozzan T. Pathways of Ca2+ influx at the plasma membrane: voltage-, receptor-, and second messenger-operated channels. Exp. Cell. Res. 1987; 171:271-283.
3. Montell C, Birnbaumer L, Flockerzi V. The TRP channels, a remarkably functional family. Cell 2002; 108:595-598.
4. Shuttleworth TJ, Thompson JL, Mignen O. ARC channels: a novel pathway for receptor-activated calcium entry. Physiology 2004; 19:355-361.
5. Furuichi T, Michikawa T, Mikoshiba K. Intracellular calcium channels. In: Calcium as a Cellular Regulator. Carafoli E, Klee C, eds. 1999. Oxford University Press, New York, pp. 200-248.
6. Endo M. Calcium release from sarcoplasmatic reticulum. Curr. Top. Membr. Transp. 1985; 25:351-384.
7. Rios E, Brum G. Involvement of dihydropyridine receptors in excitation-contraction coupling in skeletal muscle. Nature 1987; 325:717-720.
8. Nabauer M, Callewaert G, Cleemann L, Morad M. Regulation of calcium release is gated by calcium current, not gating charge, in cardiac myocytes. Science 1989; 244:800-803.
9. Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science 1993; 262:740-744.
10. Lipp P, Egger M, Niggli E. Spatial characteristics of sarcoplasmic reticulum Ca2+ release events triggered by L-type Ca2+ current and Na+ current in guinea-pig cardiac myocytes. J. Physiol. 2002; 542:383-393.
11. Tsugorka A, Rios E, Blatter LA. Imaging elementary events of calcium release in skeletal muscle cells. Science 1995; 269:1723-1726.
12. Parker I, Ivorra I. Localized all-or-none calcium liberation by inositol trisphosphate. Science 1990; 250:977-979.
13. Yao Y, Choi J, Parker I. Quantal puffs of intracellular Ca2+ evoked by inositol trisphosphate in Xenopus oocytes. J. Physiol. 1995; 482:533-553.
14. Miyakawa T, Maeda A, Yamazawa T, Hirose K, Kurosaki T, Iino M. Encoding of Ca2+signals by differential expression of IP3 receptor subtypes. 1. EMBO J. 1999; 18:1303-1308.
15. Iino M. Biphasic Ca2+ dependence of inositol 1,4,5-trisphosphate- induced Ca2+ release in smooth muscle cells of the guinea pig taenia caeci. J. Gen. Physiol. 1990; 95:1103-1122.
16. Finch EA, Turner TJ, Goldin SM. Calcium as a co-agonist of inositol 1,4,5 trisphosphate-induced calcium release. Science 1991; 252:443-446.
17. Strehler EE, Zacharias DA. Role of alternative splicing in generating isoform diversity among plasma membrane calcium pumps. Physiol. Rev. 2001; 81:21-50.
18. Quednau BD, Nicoll DA, Philipson KD. The sodium/calcium exchanger family-SLC8. Pflugers. Arch. 2004; 447:543-548.
19. MacLennan DH, Green NM. Structural biology. Pumping ions. Nature 2000; 405:633-634.
20. Dolmetsch RE, Pajvani U, Fife K, Spotts JM, Greenberg ME. Signaling to the nucleus by an L-type calcium channel-calmodulin complex through the MAP kinase pathway. Science 2001; 294: 333-339.
21. Hardingham GE, Arnold FJL, Bading H. A calcium microdomain near NMDA receptors: on switch for ERK-dependent synapse-to- nucleus communication. Nature Neurosci. 2001; 4:565-566.
22. Jaffe LF. Classes and mechanisms of calcium waves. Cell Calcium 1993; 14:736-745.
23. Gaspers LD, Thomas AP. Calcium signaling in liver. Cell Calcium 2005; 38:329-342.
24. Nakayama S, Kawasaki H, Kretsinger R. Evolution of EF-hand proteins. In: Calcium Homeostasis. Carafoli E, Krebs J, eds. 2000. Springer-Verlag, Berlin, pp. 30-58.
25. Schwaller B, Meyer M, Schiffmann S. ‘New’ functions for ‘old’ proteins: the role of the calcium-binding proteins calbindin D-28k, calretinin and parvalbumin, in cerebellar physiology. Studies with knockout mice. Cerebellum 2002; 1:241-258.
26. Racay P, Gregory P, Schwaller B. Parvalbumin deficiency in fast-twitch muscles leads to increased ‘slow-twitch type’ mitochondria, but does not affect the expression of fiber specific proteins. FEBS J. 2006; 273:96-108.
27. Brini M. Ca2+ signalling in mitochondria: mechanism and role in physiology and pathology. Cell Calcium 2003; 34:399-405.
28. Braun AP, Schulman H. The multifunctional calcium/calmodulin- dependent protein kinase: from form to function. Annu. Rev. Physiol. 1995; 57:417-445.
29. De Koninck P, Schulman H. Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations. Science 1998; 279:227-230.
30. Oancea E, Meyer T. Protein kinase C as a molecular machine for decoding calcium and diacylglycerol signals. Cell 1998; 95:307-318.
31. Violin JD, Zhang J, Tsien RY, Newton AC. A genetically encoded fluorescent reporter reveals oscillatory phosphorylation by protein kinase C. J. Cell. Biol. 2003; 161:899-909.
32. Cullen PJ. Decoding complex Ca2+ signals through the modulation of Ras signaling.Curr. Opin. Cell. Biol. 2006; 18:157-161.
33. Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu. Rev. Neurosci. 2002; 25:103-126.
34. Liu Q, Walker SA, Gao D, Taylor JA, Dai YF, Arkell RS, Bootman MD, Roderick HL, Cullen PJ, Lockyer PJ. CAPRI and RASAL impose different modes of information processing on Ras due to contrasting temporal filtering of Ca2+. J. Cell. Biol. 2005; 170:183-190.
35. White BA. Evidence for a role of calmodulin in the regulation of prolactin gene expression. J. Biol. Chem. 1985; 260:1213-1217.
36. West AE, Chen WG, Dalva MB, Dolmetsch RE, Kornhauser JM, Shaywitz AJ, Takasu MA, Tao X, Greenberg ME. Calcium regulation of neuronal gene expression. Proc. Natl. Acad. Sci. U.S.A. 2001; 98:11024-11031.
37. Crabtree GR. Calcium, calcineurin, and the control of transcription. J. Biol. Chem. 2001; 276:2313-2316.
38. Guerini D, Garcia-Martin E, Gerber A, Volbracht C, Leist M, Merino CG, Carafoli E. The expression of plasma membrane Ca2+ pump isoforms in cerebellar granule neurons is modulated by Ca2+. J. Biol. Chem. 1999; 274:1667-1676.
39. Guerini D, Wang X, Li L, Genazzani A, Carafoli E. Calcineurin controls the expression of isoform 4CII of the plasma membrane Ca2+ pump in neurons. J. Biol. Chem. 2000; 275:3706-3712.
40. Li L, Guerini D, Carafoli E. Calcineurin controls the transcription of Na+/Ca2+ exchanger isoforms in developing cerebellar neurons. J. Biol. Chem. 2000; 275:20903-20910.
41. Genazzani AA, Carafoli E, Guerini D. Calcineurin controls inositol 1,4,5-trisphosphate type 1 receptor expression in neurons. Proc. Natl. Acad. Sci. U.S.A. 1999; 96:5797-5801.
42. Graef IA, Mermelstein PG, Stankunas K, Neilson JR, Deisseroth K, Tsien RW, Crabtree GR. L-type calcium channels and GSK-3 regulate the activity of NF-ATc4 in hippocampal neurons. Nature 1999; 401:703-708.
43. Carrion AM, Link WA, Ledo F, Mellstrom B, Naranjo JR. DREAM is a Ca2+-regulated transcriptional repressor. Nature 1999; 398:80-84.
44. Gomez-Villafuertes R, Torres B, Barrio J, Savignac M, Gabellini N, Rizzato F, Pintado B, Gutierrez-Adan A, Mellstrom B, Carafoli E, Naranjo JR. Downstream regulatory element antagonist modulator regulates Ca2+ homeostasis and viability in cerebellar neurons. J. Neurosci. 2005; 25:10822-10830.
45. Corneliussen B, Holm M, Waltersson Y, Onions J, Hallberg B, Thornell A, Grundstrom T. Calcium/calmodulin inhibition of basic-helix-loop-helix transcription factor domains. Nature 1994; 368:760-764.
46. Tsien RY. A nondisruptive technique for loading calcium buffers and indicators into cells. Nature 1981; 290:527-528.
47. Inouye S, Noguchi M, Sakaki Y, Takagi Y, Miyata T, Iwanaga S, Miyata T, Tsuji FI. Cloning and sequence analysis of cDNA for the luminescent protein aequorin. Proc. Natl. Acad. Sci. U.S.A. 1985; 82:3154-3158.
48. Rizzuto R, Brini M, Murgia M, Pozzan T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighbouring mitochondria. Science 1993; 262:744-747.
49. Marsault R, Murgia M, Pozzan T, Rizzuto R. Domains of high Ca2+ beneath the plasma membrane of living A7r5 cells. EMBO J. 1997; 16:1575-1581.
50. Pinton P, Pozzan T, Rizzuto R. The Golgi apparatus is an inositol 1,4,5-trisphosphate-sensitive Ca2+ store, with functional properties distinct from those of the endoplasmic reticulum. EMBO J. 1998; 15:5298-5308.
51. Miyawaki A, Llopis J, Heim R, McCaffery JM, Adams JA, Ikura M, Tsien RY. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature 1997; 388:882-887.
52. Romoser VA, Hinkle PM, Persechini A. Detection in living cells of Ca2+-dependent changes in the fluorescence emission of an indicator composed of two green fluorescent protein variants linked by a calmodulin-binding sequence. A new class of fluorescent indicators. J. Biol. Chem. 1997; 272:13270-13274.
53. Heim N, Griesbeck O. Genetically encoded indicators of cellular calcium dynamics based on troponin C and green fluorescent protein. J. Biol. Chem. 2004; 279:14280-14286.
54. Palmer AE, Giacomello M, Kortemme T, Hires SA, Lev-Ram V, Baker D, Tsien RY. Ca2+ indicators based on computationally redesigned calmodulin-peptide pairs. Chem. Biol. 2006; 13:521-530.
Further Reading
Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000; 1:11-21.
Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003; 4:517-529.
Carafoli E, Santella L, Branca D, Brini M. Generation, control, and processing of cellular calcium signals. Crit. Rev. Biochem. Mol. Biol. 2001; 36:107-260.
Carafoli E. The calcium-signalling saga: tap water and protein crystals. Nat. Rev. Mol. Cell Biol. 2004; 4:326-332.
Carafoli E. Calcium-mediated cellular signals: a story of failures. Trends Biochem. Sci. 2004; 29:371-379.
Carafoli E, Genazzani A, Guerini D. Calcium controls the transcription of its own transporters and channels in developing neurons. Biochem. Biophys. Res. Commun. 1999; 266:624-632.
Rizzuto R, Pozzan T. Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol. Rev. 2006; 86:369-408