CHEMICAL BIOLOGY
Lipidated Peptide Synthesis
Marc Gerauer, Sebastian Koch and Herbert Waldmann, Max-Planck-Institut fur Molekulare Physiologie and Universitat Dortmund, Dortmund, Germany
Luc Brunsveld, Max-Planck-Institut fur Molekulare Physiologie and Chemical Genomics Centre, Dortmund, Germany
doi: 10.1002/9780470048672.wecb290
In this review, an overview is given for the synthesis of lipidated peptides such as those belonging to the Rab, Ras, eNOS, and hedgehog proteins. The different approaches for the synthesis of specific lipidated peptides (palmitoylated, prenylated, and myristoylated) are discussed with special emphasis on solid-phase synthesis methods, because these methods have turned out to be the preferred synthesis method for most required peptides. Different solid-phase methods are described that are individually suited for different types of lipidated peptides, differing, for example, in lipidation pattern or amino acid side-chain functionality and in their protein ligation functionality. For the described solution approaches, the block coupling strategies followed for the different lipidated peptides are highlighted. A special section in this review discusses the different synthetic tools for the incorporation of the lipid functionalities in the peptides. Although a generally validated strategy for the synthesis of lipidated peptides does not exist, because of the large number of different functionalities, this review aids in the conceptual design of synthetic protocols for lipidated peptides. Finally, the methods for the ligation of lipidated peptides to proteins are shortly discussed, as these methods also significantly influence the design and synthesis of lipidated peptides.
The continued existence of cells crucially depends on their ability to receive signals from the environment and to respond to them in a suitable way. This process of signal transduction, i.e., the forwarding of signals from the extracellular matrix via the cytoplasm into the nucleus, involves, among others, proteins that are modified with structural hydrophobic features attached to the peptide backbone. Posttranslational lipidation by acylation with fatty acids, alkylation with prenyl moieties, and conjunction of more complex lipid components, for instance, the glycosylphosphatidylinositol (GPI) moiety, occurs on a wide variety of intracellular and extracellular signaling proteins. An example of proteins containing lipid moieties are membrane-attached proteins. This review provides a concise overview of the synthesis of lipidated peptides from these membrane proteins and discusses possible methods for generating intact proteins with these peptides. The family of Ras proteins is one of the most relevant examples of lipidated membrane proteins from both their abundance and their biological and pharmaceutical importance and is therefore used as a general motif in this review (1).
General Considerations For Lipidated Peptide Synthesis
Five predominant lipidation motifs can be found on proteins, resulting in increased complexity from the stable and simple myristoyl group that can be found on N-terminal glycines to the GPI anchor featuring, apart from the lipid group, a complex sugar motif and phosphate groups (Fig. 1). The three most common types of posttranslational lipid modifications encountered in the Ras superfamily, for example, are N-myristoylation, S-palmitoylation, and S-isoprenylation (2-4), of which palmitoylation is the only reversible one (5). These lipid modifications are important for the correct biological function of Ras proteins, as they require the localization to the inner leaflet of the plasma membrane and are generally found at the C-terminus of the protein. As for other lipidated proteins, until recently, most experiments with these proteins, such as structure determination by X-ray diffraction, nuclear magnetic resonance (NMR), and biochemical characterization, were performed on the soluble part of the proteins, i.e., the protein without the C-terminus. The results obtained via these approaches can, however, at best only give an approximation of the real situation, especially because diversity between the individual members is often encountered in the lipidated part of the protein. The biochemical generation of fully functionalized and modified lipidated proteins is, however, difficult and time-consuming. In the case of S-palmitoylation, it leads to heterogeneous mixtures and is, therefore, in most cases, not practical or applicable. Therefore, in recent years, chemical biological approaches have been developed that give access to fully functional lipidated peptides and proteins, together with additional non-natural modifications, which was achieved via two techniques that were developed more or less in parallel: 1) the progress in the field of protein ligation and chemical synthesis of proteins (6) and 2) the progress in the field of lipidated peptide synthesis, both in solution and on solid support. The development of these synthetic methods with special attention to the solid-phase methods are the focus of this review. Synthetic strategies giving access to lipidated peptides have been developed during the last 10 years and have been reviewed both for solution and for solid support approaches (7, 8). This review is therefore not an all-inclusive review, but it highlights how different types of lipidated peptides can be most effectively synthesized.

Figure 1. Overview of different lipidation motifs found on membrane binding proteins.
The synthesis of lipidated peptides can follow different strategies such as the use of solid-phase versus solution-phase techniques, the use of lipidated building blocks versus peptide lip- idation, and the application of the Boc versus Fmoc strategy as well as enzymatic (9-12) and noble metal (13) sensitive methods. The decision regarding which strategy to follow will generally depend on the types and positions of the lipid groups in the peptides, the length of the peptide, the C-terminal functionality, the presence of additional functional groups such as fluorescent markers or spin labels, and the purification strategy. Particularly regarding the lipids and functional groups, the synthetic strategy has to be fully adapted to the reactivities of these modifications. For solid-phase chemistry, the choice of the linker to the resin is an additional criterion to consider in the synthetic planning (Fig. 2).
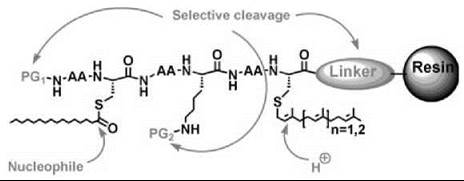
Figure 2. Characteristic reactivities of lipidated peptides and requirements for an appropriate solid-phase synthesis protocol with protecting groups and linker orthogonal to each other and to the lipid functionalities.
For lipidated peptides, some general guidelines have emerged: 1) (strong) Acid-labile protecting groups or linkers cannot be used in combination with prenyl groups. 2) Prenyl groups are not compatible with protecting groups that have to be removed under hydrogenolytic conditions. 3) Thioesters such as palmitoyl groups attached to cysteines are labile toward nucleophiles. 4) Non-natural functional groups such as fluorophores, photoactivatable groups, and tags for ligation to proteins generally impose synthetic limitations. 5) Prenylated dipeptide esters undergo rapid diketopiperazine formation upon N-terminal deprotection. 6) The palmitoyl group on an N-terminally unmasked cysteine rapidly undergoes an undesired S,N-acyl shift.
An additional issue that requires attention during the synthetic process is the purification of the lipidated peptides. Both final products and intermediates are often badly soluble in both water and organic solvent or feature detergent-like characteristics, which limits purification via both silica gel chromatography and reversed-phase high-performance liquid chromatography (HPLC). Purification can therefore be time-consuming and can result in low yields. However, the strongly dissimilar nature of the coupling partners, for instance, with respect to hydrophobicity, can also be used to facilitate the separation of product and starting compounds or the separation of double-lipidated from single-lipidated peptide.
Considering these features and limitations, it is not surprising that a general strategy for the synthesis of lipidated peptides, such as the Fmoc- or Boc-strategy for normal peptides, does not exist. However, several tools have been developed such as protecting groups, resin linkers, and synthetic approaches that have allowed some general strategies to emerge with which most lipidated peptides can be synthesized. This review tries to guide the reader to some generally validated approaches that meet the restraining demands imposed by the peptides. In our experience, solid-phase approaches offer fast and flexible entries to multiply lipidated peptides; therefore, the main focus will be on these solid-phase approaches.
Introduction of Lipids Into Peptides
Introduction of lipid functionalities into peptides can follow two general strategies, independent of solid-phase or solution-phase chemistry. Either the lipid groups are introduced via coupling of prelipidated amino acids to the peptide (Fig. 3a) or they are introduced via selective lipidation of a peptide (Fig. 3b-e). Both the use of prelipidated building blocks (14-16) and of resin lipidation (17-25) have been investigated for the synthesis of lipidated peptides on solid support.
On-bead lipidation
Lipidation on the solid support requires an orthogonal protecting group strategy that allows the stepwise introduction of different lipid groups on the same peptide such as a farnesyl group and a palmitoyl group (Fig. 3c) (20, 21). This approach generally uses a large excess of the lipid groups, which becomes problematic if the lipid groups need to be synthesized (e.g., in modified form) and requires an extensive protecting group strategy. A possible solution to overcome these problems might be the application of the recently developed reversed approach of S-farnesylation and S-palmitoylation to solid-phase chemistry (26). In this approach, lipid groups are introduced via the SN2-displacement of a bromide by reaction of a thiol group containing lipid as nucleophile with bromoalanine-containing peptides as electrophile in solution (Fig. 3b). Another elegant approach to introduce a prenyl thioether into peptides could be the use of a chemoselective conjugate addition of an thiolate nucleophile (e.g., farnesylthiolate) to a dehydroalanine (Fig. 3d) (27). The absence of stereoselective control in this conjugate addition can be solved using aziridine-2-carboxylic acid-containing peptides (Fig. 3e) (28). These newly developed methods for the incorporation of lipi- dated amino acids might prove beneficial in future solid-phase lipidated peptide synthesis.
Prelipidated amino acid building blocks
An approach to overcome the issues associated with on-resin prenylation for the synthesis of lipidated peptides relies on the use of prelipidated cysteine building blocks. These prelipidated building blocks can be handled as normal amino acids in the coupling sequence and can be inserted anywhere in a sequence without the need to modify the protecting group strategy (Fig. 3a). This approach thus provides a highly flexible and generic entry to lipidated peptides (15).

Figure 3. Different approaches for the introduction of lipid functionalities, here exemplified via the farnesyl group, into peptides. (A) Lipidated amino acid building blocks. (B) Substitution of bromoalanine with a nucleophile. (C) Alkylation or acylation of a free thiol functionality of a cysteine. (D) Conjugate addition of a nucleophile (e.g., farnesylthiolate) to a dehydroalanine. (E) Conjugate addition of a nucleophile to aziridine-2-carboxylic acid containing peptides.
Synthesis of Lipidated Peptides
Lipidated peptides can be synthesized both in solution and on solid support. The recent focus on lipidated peptide synthesis has shifted to the preparation of lipidated peptides on solid supports as it intrinsically offers faster and more flexible entries to the target peptides (15). Issues that should be considered for solid-phase lipidated peptides synthesis are, for example, the screening of different linkers and cleavage conditions as well as evaluation of synthesis routes for the introduction of the lipid groups with a suitable orthogonal protecting group strategy. By analogy to the solution-phase approaches, protecting groups must be orthogonal to the lipids and eventually other functional groups in the peptides. Additionally, the linker to the resin should be cleavable under conditions not interfering with the peptide functionalities. In an ideal setup, the cleavage of the linker would also allow for the introduction of different types of functional groups at the C-terminal carboxyl function.
Here, both solution-phase approaches and solid-phase approaches undertaken for the synthesis of specific lipidated peptides are reviewed (Fig. 4). For the solution-phase approaches, the focus is on the coupling strategy applied, i.e., which block couplings were pursued. The solid-phase approaches are reviewed with the focus on the type of linker to be used for specific types of lipidated peptides.

Figure 4. Sequences of lipidated peptides discussed in this review. The frizzled lines indicate the building blocks used for the block coupling strategy of the solution-phase synthesis (when applicable). Abbrevations: BiotAca: N-(+)-Biotinyl-6-aminocaproyl; MantAca: N-methylanthranyl-6-aminocaproyl; MIC: 6-Maleimidocaproyl; NBDAca: N-(4-nitrobenz-2-oxa-1,3-diazol-7-yl)-6-aminocaproyl.
Small lipidated model peptides for biophysical investigations
Small cysteine-containing peptides similar to sequences of S-acylated proteins were generally synthesized in solution. The tetrapeptide model Bimane-SC(Sf Bu)RC(Far)OMe representative for the carboxy terminus of H-Ras and featuring the Bimane fluorophore was prepared in solution using Fmoc-chemistry, introducing the farnesyl group at the stage of the Fmoc-protected dipeptide (29). The acylated peptides (Myr)GCX-Bimane (X = G, L, R, T, V), which are found in certain nonreceptor tyrosine kinases and α-subunits of several heterotrimeric G-proteins, were synthesized in solution using common solution-phase peptide synthesis with N-myristoylglycine as the building block (29). These model peptides were acylated with Palmitoyl-CoA in phospholipid vesicles at physiological pH. For such uncatalyzed spontaneous reactions, only a modest molar excess of acyl donor species (2.5:1) was necessary. Unprotected side chains of threonine or serine do not interfere with this S-acylation.
Lipidated peptides incorporating the C(GerGer)XC(GerGer)OMe motif found in several Rab and homologous proteins were also synthesized in solution via Fmoc-chemistry following cysteine deprotection and geranylgeranylation (30).
C-terminal lipidated peptide of the influenza virus hemagglutinin A
The influenza virus hemagglutinin A contains a lipidated peptide fragment at the C-terminus featuring two palmitoylated cysteines and two amino acids with a polar side chain. This peptide has been synthesized in solution and the strategy for this synthesis was based on the fragment condensation of the lipidated tetrapeptide TIC(Pal)I that was coupled N-terminal to the palmitoylated dipeptide RC(Pal) and, after deprotection, elongated with NBD-aca-labeled methionine (31). For this block coupling strategy, a set of three orthogonal protecting groups was required, whereby the use of base/nucleophile-labile and hydrogenolytically removable protecting groups was not permitted. The use of the acid labile Boc group for the N-terminus, the enzymatically removable PAOB ester for the C -terminus, and the Pd0-sensitive Alloc group for the arginine side-chain function turned out to be a successful combination.
C-terminal lipidated peptides of H-/N-Ras
A large body of work has been devoted to the synthesis of the C-terminal lipidated peptides of the small GTPases H-/N-Ras. As such, these peptides have been synthesized in solution, via combined solution and solid support approaches and completely on solid phase.
For the synthesis of a small library of palmitoylated and isoprenylated N-Ras peptides in solution, a modular strategy was adopted, with the tetrapeptide MGLP as the key intermediate. This tetrapeptide intermediate allowed for further elongation at its C-terminus with lipidated or nonlipidated cysteine methyl esters, as well as the addition of various N-terminal maleimi-docaproyl (MIC)-labeled dipeptides, consisting of different GC lipidated units (32, 33). The synthesis was performed under common conditions using the Fmoc, Boc, and Alloc protecting group strategy. Using this methodology, a number of N-Ras derivatives containing natural and nonnatural lipid residues were produced, and the technique was extended to also include a number of fluorescent derivatives.
In a reversed approach, N-Ras lipidated peptides were synthesized in solution via an SN2 displacement of bromoalanine containing hexapeptides with thiopalmitic acid and farnesylmer-captane as nucleophiles (26). The synthetic route started with the dipeptides and tetrapeptides A(Br)M and GLPA(Br), both incorporating bromoalanine. After farnesylation of the C-terminal tetrapeptide, it was coupled to the N-Alloc protected dipeptide. This farnesylated hexapeptide was then treated with thiopalmitic acid for final palmitoylation.
The key feature of the solution synthesis of the C-terminal octapeptide of H-Ras containing one farnesyl thioether and two palmitoyl thioester moieties is the orchestration of the acid-labile tert-butyl ester function as carboxy protecting group, the Pd0-sensitive Alloc function as amino-blocking group, and the reduction-labile tert-butyl disulfide function for masking of thiol groups (13). In addition, a serine hydroxyl and, in particular, a lysine e-amino group are located in the vicinity of the thioester groups, increasing the danger of S-O and S-N acyl migrations in the course of the synthesis. The assembly in solution was achieved by dividing the triply lipidated peptide into the two selectively C- and N-terminal unmasked palmitoylated tripeptide building blocks PGC(Pal) and MSC(Pal), the N-terminally unmasked lysine derivative, and an S-farnesylated cysteine methyl ester.
In a combined solution-phase and solid-phase approach the N-Ras lipidated peptide was synthesized by coupling the C-terminal farnesylated cysteine methylester in solution to the rest of the peptide that had been assembled on solid support (34). The second cysteine was protected as a tert-butyl disulfide, thus allowing reductive cleavage under physiological conditions, and the N-terminal amine of the peptide was connected to a maleimido group for protein ligation purposes. The use of these protecting and functional groups made the final segment condensation a relatively straightforward task. An important issue that has to be kept under scrutiny when performing such segment condensations is the possibility of racemization, the selection of validated coupling techniques that avoid the use of basic conditions and polar solvents, however, has usually proved sufficient to avoid or minimize this racemization. Typically, the amino acid (4 equiv) is treated with DIC (4 equiv) and an excess of HOBt (6 equiv) in DMF/CH2Cl2 mixtures.
For the complete solid-phase synthesis of H- and N-Ras peptides, the hydrazide linker turned out to be the linker of choice (14). This linker is cleaved by oxidation to an acyldiazene that is then attacked by a suitable nucleophile. The linker is orthogonal to classic urethane protecting groups such as Boc, Fmoc, and Alloc, and racemization does not occur on cleavage. Typical nucleophiles that can be used for the cleavage are amines, water, and alcohols (35). The oxidation sensitivity of the linker does require the coupling reactions, and especially Fmoc deprotecting reactions, to be performed under exclusion of oxygen.
The hydrazide linker allowed, for example, the synthesis of a completely lipidated N-Ras peptide sequence, including an additional fluorophore attached to a lysine side chain or N-terminal glycine and a C-terminal methyl ester (Fig. 5) (14). Coupling conditions of the amino acids were similar to normal solid-phase peptide synthesis (SPPS) with other linkers. One important precaution was taken, however, to avoid an S-N acyl shift of the palmitoyl group after the Fmoc deprotection of the palmitoylated cysteine. It was found that the S-palmitoylated peptides remained stable when the Fmoc deprotection was performed using a solution of 1% DBU in DMF for 2 x 30 seconds. The next coupling was then performed immediately with preactivated amino acid and HATU as a coupling reagent. Using similar approaches, many different peptides of the Ras family were synthesized on the hydrazide linker, including those containing farnesyl, geranylgeranyl, fluorescent labeled geranyl, and palmitoyl lipids. Fluorescent markers such as NBD and photoactivatable groups like the benzophenone group have been introduced and Ras superfamily peptides with C -terminal acid, ester, or amide functionalities have been cleaved from the resin. Similar solid-phase results for Ras peptides could be obtained with another linker, the Ellman sulfonamide linker (vide supra) (36).
An orthogonal linker does not alleviate the use of protecting groups for the amino acid side-chain functionalities. Therefore, for peptides synthesized on the hydrazide linker in general, protecting groups were used that were either highly acid labile such as the trityl group for alcohols, base labile such as the 9-fluorenylmethyl ester group for the protection of carboxylic acids, or labile toward noble metals such as the Alloc group for amines and allyl ester for acids (15). These protecting groups can be cleaved from the peptide while still on the resin. However, amines are preferably not deprotected before cleavage as their nucleophilic nature gives rise to side products; the liberated amine can attack the oxidized linker and cleave the peptide, leading to cyclic peptides or oligomers. The more sterically demanding and less nucleophilic alcohol functions of, for example, the serine side-chains feature these problems to a much lesser extent and can generally be deprotected on the resin. Having the amine functionalities protected after cleavage thus requires an additional deprotection step in solution. In general, the synthesis of lipidated peptides on the hydrazide linker is successful for peptides with up to 10 amino acids. As such, the hydrazide linker is one of the preferable systems for a broad range of solid-phase lipidated peptide synthesis.
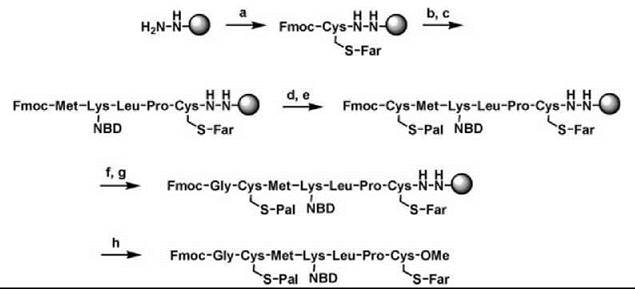
Figure 5. Solid-phase synthesis of an N-Ras lipidated peptide on hydrazide resin. (A) Fmoc-Cys(Far)-OH, HBTU, HOBt, TMP, CH2CI2/DMFS (1:1). (B) piperidine/DMF (1:4). (C) Fmoc-AA-OH, HBTU, HOBt, DIPEA, DMF. (D) piperidine/DMF (1:4). (E) Fmoc-Cys(Pal)-OH, HBTU, HOBt, TMP, CH2Cl2/DMF (1:1). (F) DBU (1 %) in DMF. (G) Fmoc-Gly-OH, HATU (5 eq.), DIPEA (20 eq.) CH2Cl2/DMF (7:1). (H) Cu(OAc)2 (0.5 eq.), pyridine (30 eq.), acetic acid (50 eq.), methanol (215 eq.) CH2Cl2, O2.
Polybasic prenylated C-terminal lipidated peptides
The synthesis of polybasic isoprenylated lipidated peptides, such as the C-termini of K-Ras4B, D-Ral, and Rho A, have been most successful on the solid support. The solution strategies to the peptides, generally through fragment condensation, were difficult because of, for example, different polarities and solubilities of the fragments.
The solution synthesis of the polybasic C-terminus of Rho A required coupling of a polybasic KKK tripeptide with the SGC(GerGer) tripeptide containing the geranylgeranylated cysteine methylester and an N-terminal coupling with a fluorescent labeled glycine (37). For this approach, the orthogonallity of the Fmoc, Alloc, and OtBu protecting group was applied. The same methodology for the synthesis of the polybasic K-Ras4B peptide failed, however, because of the low coupling yields resulting from the different solubility properties of the fragments.
The methods of choice for the synthesis of polybasic lipidated peptides such as those corresponding to the C-termini of RhoA, K-Ras4B, and D-Ral are solid-phase approaches using the trityl linker or the chlorotrityl linker (37, 38). The mildly acidic cleavage conditions of these resins are still orthogonal to the farnesyl moiety and often also to the geranylgeranyl moiety. Attachment of the peptide via the C-terminus would limit the C-terminal functionality to a free carboxylic acid. Therefore, the peptide can generally be attached via a lysine side chain near the C-terminus (37, 38), which avoids troublesome block condensations on the solid support with concomitant risk of racemization. The C-terminal prenylated cysteine of the peptide is introduced by coupling a prelipidated cysteine to the selectively deprotected carboxyl function of the anchored lysine. After obtaining such a dipeptide on the resin, the additional steps of the synthesis can be performed in the N-terminal direction, using standard protocols and, for example, an Fmoc/Alloc strategy. The Alloc protected side chains of lysine and arginine can be liberated on the solid support via cleavage with palladium(0) and an appropriate nucleophile. Treatment of the resin with only 1% TFA and a scavenger subsequently releases the peptide while leaving the prenyl/geranylgeranyl group intact (38). A critical requirement for the use of the trityl linker in the above-described method is the presence of an anchor group in the C-terminal part of the peptide sequence that allows cleavage from the resin with 1% TFA only. Another current limitation to the method is the incompatibility with thioesters, for example, palmitoylated cysteines. The deprotection of the Alloc groups with piperidine as scavenger is not compatible with the nucleophile-labile thioester. Optimization of the type of nucleophile might provide a solution, but deprotection of a large number of Alloc groups on solid support is not always a trivial matter. Finally, the Alloc deprotection is incompatible with the maleimide functionality as phosphines, used as ligands for the palladium catalyst, undergo nucleophilic addition to this functionality. Nevertheless, for polybasic prenylated peptides, the use of the trityl linker is a reliable solid-phase approach.
C-terminal lipidated rab peptides
The synthesis of double geranylated peptides representing the Rab C-termini, for example the Rab7 C-terminus, has also been performed both in solution and on solid support. In general, the bivalent character of these peptides, featuring both highly hydrophobic lipid groups and polar amino acids, makes them difficult to handle. The synthesis in solution does not allow assembly of the hexapeptide by N-terminal linear elongation, for example, because of undesired diketopiperazine formation at the dipeptide stage (16). Therefore, a block-coupling method was chosen, with the dipeptide Fmoc-SC(GerGer) as a general building block (15). The dipeptide was elongated by C-terminal coupling with serine allylester. After deprotection of the allyl ester, the tripeptide was coupled to a geranylgeranylated cysteine methyl ester. N-Terminal elongation of the tetrapeptide to the desired hexapeptide was performed again via a block coupling, this time with the dipeptide Fmoc-C(StBu)E(Fm), to introduce a non-native cysteine that allows for ligation of the peptide to the protein. By employment of the Fmoc protecting group for the N-terminal amino function and the Fm protecting group for the side-chain carboxylic acid functionality of glutamic acid, a system was generated that allowed simultaneous cleavage of both protecting groups under basic conditions in a final step.
The solid-phase synthesis of lipidated Rab peptides has relied on the hydrazide linker, similar to the lipidated Ras peptides (15). By employing geranylgeranylated or fluorescent labeled lipidated cysteines as building blocks, a highly flexible straightforward strategy with no extravagant expenses or complex protecting group chemistry was developed. For the peptide assembly standard Fmoc chemistry was used. The side-chain protecting groups O-Trt and COOAll were subsequently removed on the resin to leave only the N-terminal amine and thiol functionalities masked. The usage of different nucleophiles for the cleavage of the peptide from the hydrazine linker allowed the introduction of a variety of functional groups at the C-terminus.
Lipidated peptides of endothelial NO-synthase
The double palmitoylated and N-terminal myristoylated 29-mer lipidated peptide of eNOS has been synthesized both via a combined solution/solid-phase approach and via solid-phase peptide chemistry alone. For the combined technique, a combination of enzyme-labile, acid sensitive, and noble metal sensitive protecting groups was chosen for the solid-phase synthesis, combined with fragment condensation in solution (39). The eNOS peptide was divided into the N-myristoylated decapeptide MyrGN-LKSVGQEP, the 5 -palmitoylated pentapeptide GPPC(Pal)G, the octapeptide (LG)4, and the S-palmitoylated hexapeptide LC(Pal)GKQG. For the synthesis and selective deprotection of the S -palmitoylated building blocks, the enzyme labile para-phenyl-acetoxybenzyloxycarbonyl urethane group and the Pd(0)-labile allyl ester were chosen as temporary N-terminal and C-terminal protecting functions. The side chains of Asn, Lys, Ser, Gln, and Glu were masked with acid-labile protecting groups to be cleaved off simultaneously in the final step of the synthesis. The entire 29-mer was finally assembled in solution by appropriate fragment condensation.
The eNOS lipidated peptide was also assembled on solid support, for which the Ellman sulfonamide linker was used, which also showed good results for lipidated Ras peptides (36). The linker is stable toward acid and base, activated on alkylation, but orthogonal to the methionine thioether. The target compound is released after attack of a nucleophile (40). These cleavage conditions make it orthogonal to the lipid functionalities and a good tool for the synthesis of lipidated peptides. In some cases, the Ellman sulfonamide linker seems to give even better results than the hydrazide linker, especially for long peptides with multiple lipid motifs. The double palmitoylated and N-terminal myristoylated 29-mer eNOS peptide is such a case. Using prelipidated building blocks the peptide could be easily assembled and obtained after cleavage. The example of the eNOS peptide shows that with good design, both the combined solution/solid-phase approach and the solid-phase approach enable excess to difficult lipidated peptides. The solid-phase approach, however, has the advantage of being significantly faster and easier.
Lipidated peptides of hedgehog proteins
The C-terminal steroid modified heptapeptides, corresponding to the C-terminus of Hedgehog proteins, were synthesized in a combined solid-phase/solution approach (41). The synthesis built on the dipeptide Fmoc-SG-OAll that was connected to the trityl resin via the free hydroxyl functionality of the serine. The C-terminal allyl ester was cleaved by noble-metal-mediated allyl transfer to phenylsilane, and the carboxylic acid was connected to glycine-steroid esters by employing PyBOP as a coupling reagent. Subsequently, the N-terminal peptide chain elongation was achieved by means of standard Fmoc solid-phase peptide chemistry to yield different fluorescent labeled peptides carrying an NBD group at a lysine side chain or at the N-terminus or MIC-modified peptides. They were cleaved from the resin under mild conditions with 5% TFA, furnishing the desired products in high yields without any side reactions.
Model transmembrane peptides
A palmitoylated model transmembrane peptide consisting of a tryptophane-flanked poly-Leu-Ala peptide sequence similar to gramicidin A has been synthesized on solid support (22). It is an interesting example displaying the possible optimization protocol that has to be undertaken with lipidated peptide synthesis. According to the experience of reported lipidated peptide synthesis, a building block strategy was chosen. The peptide was synthesized on a Rink amide linker. In the final step of the solid-phase peptide synthesis, the coupling of the palmitoylated N-acetyl cysteine building block was not feasible. Aminolysis of the thioester was much faster than the carbodiimide-mediated peptide coupling. Introduction of the palmitoyl group via on-bead lipidation of the cysteine required deprotection of the S–tert-butylsulfanyl protected side chain. The standard deprotection reagents P(Bu)3in NMP/H2O, however, led to desulfurization of the cysteine, resulting in an alanine-containing peptide instead of an palmitoylated N-acetyl cysteine. The explanation for this nearly quantitative reaction might reside in the hydrophobicity of the peptide. The phosphonium-S-cysteine moiety is thus not able to react with H2O and β-elimination mediated by the tert-butyl thiolate is the favored step. The S-(4-methoxytrityl)cysteine derivative proved to be the building block of choice, as it could be deprotected under mild acidic conditions, orthogonal to the other protecting groups in the peptide, which allowed for the lipidation of the cysteine and the synthesis of palmitoylated transmembrane model peptide.
Synthesis of Lipidated Proteins Using Lipidated Peptides
Production of recombinant proteins is a key technology in the life sciences. However, progress in generation of authentic or engineered polypeptides has been more difficult for proteins that undergo posttranslational modifications, such as lipidation. This imbalance is primarily a result of the intricacy of protein modification pathways as well as the lack of methodologies for their manipulation (42). Studies of lipidated proteins require methods that provide preparative amounts of protein, both with natural and with new functionalities such as fluorescence, photoreactivity, spin-labeled groups, or lipid groups at non-native positions. The recently developed protein ligation methods provide the necessary platform for combining large recombinant protein scaffolds with peptides generated by organic synthesis (43-46).
Progress in the area of protein ligation methods has given access to a broad spectrum of methods for the semisynthetic production of posttranslationally modified proteins. These methods yield either native bonds (e.g., prior thiol capture, native chemical ligation, or expressed protein ligation) or non-native bonds (e.g., imine capture ligation, oxime ligation, or maleimidocaproic acid ligation). A variety of methods have been developed for the introduction of modified lipids and lipidated peptides into proteins. Most work in this field has been performed on Ras GTPases, for which two important approaches have been developed (Fig. 6). The first approach that incorporates synthetic lipidated peptides is based on the use of the MlC-controlled ligation (Fig. 6b) (8, 33, 34, 47, 48). This ligation requires an accessible, for example, C-terminal free thiol group on the protein, usually of a cysteine, to connect the N-terminally MIC-modified peptide. The reaction yields a non-native connection, which is, for example, in the case of Ras not problematic, because it occurs in a flexible unstructured region. Care has to be taken with this reaction that there are no other free cysteine side chains available for ligation. The second approach, Expressed Protein Ligation (EPL), connects a lipidated peptide with an N-terminal cysteine to the C-terminus of a thioester-tagged protein via a native peptide bond (Fig. 6a) (15, 16, 38, 49, 50). This reaction is in general highly selective and yields at most a cysteine mutation in the protein as non-native element. An orthogonal approach to introduce specifically prenyl moieties into proteins not relying on lipidated peptide is via an in vitro attachment of the prenyl lipids using the corresponding prenyltransferases (51). A recently explored reaction for the incorporation of lipid motifs in peptides and proteins is the copper(I)-catalyzed Huisgen’s 1,3-dipolarcycloaddition. A GPI anchor could be attached to a peptide successfully, resulting in fast uptake of the peptide into cells (52).
The protein ligation methods have been applied for the synthesis of a library of lipidated Ras GTPases with both natural modifications and non-natural modifications (Fig. 6). These lipidated Ras GTPases have served to elucidate a variety of biological questions concerning these proteins. The specific localization of Ras protein on membranes was investigated using a fluorescently labeled N-Ras protein, for example, and found to have a preference for the liquid-disordered phase over the liquid-ordered and solid-ordered phase of membranes using fluorescence studies (53). Via AFM studies, it was additionally shown that this protein is actually located in the boundary region of the domains in mixed-phase liquid-ordered/liquid-disordered bilayers. These results suggest that for N-Ras, the membrane localization is probably completely governed by the lipidated C-terminus. As such, the conformation of the C-terminus of Ras proteins bound to the membrane was investigated using NMR (54). The membrane binding of the polybasic K-Ras4B protein was also studied after protein semisynthesis (38). A photoactivatable Ras construct has been made that can be activated to selectively react with protein interaction partners (34). The functionality of these semisynthetic Ras proteins in living cells was also shown (33), enabling advanced cellular characterization of, for example, the prenylation and the palmitoylation process of the protein, using selectively functionalized proteins (55-57). Enzymatic studies using selectively addressable fluorescent Rab proteins have elucidated the geranylgeranylation process (16).
Structural studies on selectively prenylated Rab proteins have elucidated the exact interaction mechanism of Ras proteins with their transporter protein GDP dissociation inhibitor (GDI) and have led to a membrane extraction model for Rab proteins (58, 59).

Figure 6. Schematic representation of protein ligation methods applied for the introduction of a lipidated peptide on proteins and an overview of the synthesized libraries of lipidated proteins as exemplified via Ras GTPases. (A) expressed protein ligation (EPL). (B) Maleimidocaproyl (MIC).
Conclusions
Chemical biology is the application of approaches originating from chemistry and the subsequent combination of chemistry and biology to study biological phenomena previously not accessible via chemical or biological approaches alone. Lipidated peptides alone or ligated to proteins offer such entries for lipidated proteins. For such an integrated research activity, differently modified peptides and proteins that carry modifications whose structure can be changed at will through synthesis are invaluable tools. They enable experiments yielding answers in precise molecular detail hardly accessible with biological techniques alone. Therefore, the synthesis of the lipidated peptides is an important theme and is reviewed here. The reviewed examples show that the protocols for both the synthesis of new lipidated peptides and their purification cannot always be generalized. However, with the tools currently developed and appropriate design, most types of lipidated peptides can be synthesized and obtained pure. Recently developed solid-phase synthesis methods delineate the preferred strategy to access the majority of these required lipidated peptides. With the development of these solid-phase lipidated peptide synthesis techniques, the chemical tools to obtain the majority of the required proteins are now accessible.
References
1. Wittinghofer A, Waldmann H. Ras-A molecular switch involved in tumor formation. Angew. Chem. 2000; 39:4193-4214.
2. Resh MD. Fatty acylation of proteins: new insights into membrane targeting of myristoylated and palmitoylated proteins. Biochim. Biophys. Acta 1999; 1451:1-16.
3. Dunphy JT, Linder ME. Signalling functions of protein palmitoylation. Biochim. Biophys. Acta 1998; 1436:245-261.
4. Zhang FL, Casey PJ. Protein prenylation: molecular mechanisms and functional consequences. Annu. Rev. Biochem. 1996; 65:241-269.
5. Duncan JA, Gilman AG. Autoacylation of G protein alpha subunits. J. Biol. Chem. 1996; 271:23594-23600.
6. Nilsson BL, Soellner MB, Raines RT. Chemical synthesis of proteins. Annu. Rev. Biophys. Biomol. Struct. 2005; 34:91-118.
7. Pegoraro L, Moroder L. Synthesis of lipopeptides. In: Houben-Weyl Methods of Organic Chemistry, Synthesis of Peptides and Peptidomimetics. Goodman M, Felix A, Moroder L, Toniolo C, eds. 2003. Thieme, Stuttgart, Germany.
8. Kadereit D, Kuhlmann J, Waldmann H. Linking the fields—the interplay of organic synthesis, biophysical chemistry, and cell biology in the chemical biology of protein lipidation. ChemBioChem 2000; 1:144-169.
9. Schelhaas M, Glomsda S, Hansler M, Jakubke HD, Waldmann H. Enzymatic synthesis of peptides and Ras lipopeptides employing choline ester as a solubilizing, protecting, and activating group.Angew. Chem. Int. Ed. 1996; 35:106-109.
10. Nagele E, Schelhaas M, Kuder N, Waldmann H. Chemoenzymatic synthesis of N-Ras lipopeptides. J. Am. Chem. Soc. 1998; 120:6889-6902.
11. Waldmann H, Schelhaas M, Nagele E, Kuhlmann J, Wittinghofer A, Schroeder H, Silvius JR. Chemoenzymatic synthesis of fluorescent N-ras lipopeptides and their use in membrane localization studies in vivo. Angew. Chem. Int. Ed. 1997; 36:2238-2241.
12. Waldmann H, Nagele E. Synthesis of the palmitoylated and farnesylated C-terminal lipohexapeptide of the human n-ras protein by employing an enzymatically removable urethane protecting group. Angew. Chem. Int. Ed. Engl. 1995; 34:2259-2262.
13. Kadereit D, Waldmann H. Synthesis of characteristic H-Ras lipopeptides by employing noble-, metal-, acid-, and reduction-labile blocking groups. ChemBioChem 2000; 1:200-203.
14. Kragol G, Lumbierres M, Palomo JM, Waldmann H. Solid-phase synthesis of lipidated peptides. Angew. Chem. Int. Ed. 2004; 43:5839-5842.
15. Brunsveld L, Watzke A, Durek T, Alexandrov K, Goody RS, Waldmann H. Synthesis of functionalized Rab GTPases by a combination of solution- or solid-phase lipopeptide synthesis with expressed protein ligation. Chemistry 2005; 11:2756-2772.
16. Durek T, Alexandrov K, Goody RS, Hildebrand A, Heinemann I, Waldmann H. Synthesis of fluorescently labeled mono- and diprenylated Rab7 GTPase. J. Am. Chem. Soc. 2004; 126:16368-16378.
17. Joseph M, Nagaraj R. A convenient method for the synthesis of peptides acylated with palmitic acid specifically at cysteine thiol. Bioorg. Med. Chem. Lett. 1993; 3:1025-1028.
18. Pfender N, Guenin E, Greer JM, Trifilieff E. Solid-phase synthesis of a biotin-labelled thiopalmitoylated myelin proteolipid protein epitope and application in the study of uptake of antigen by macrophages. Lett. Pept. Sci. 2003; 10:581-588.
19. Denis B, Trifilieff E. Synthesis of palmitoyl-thioester T-cell epitopes of myelin proteolipid protein (PLP). Comparison of two thiol protecting groups (StBu and Mmt) for on-resin acylation. J. Pept. Sci. 2000; 6:372-377.
20. Ludolph B, Eisele F, Waldmann H. Solid-phase synthesis of lipidated peptides. J. Am. Chem. Soc. 2002; 124:5954-5955.
21. Ludolph B, Waldmann H. The synthesis of acid- and base-labile lipopeptides on solid support. Chemistry 2003; 9:3683-3691.
22. Rijkers DTS, Kruijtzer JAW, Killian JA, Liskamp RMJ. A convenient solid phase synthesis of S-palmitoyl transmembrane peptides. Tetrahedron Lett. 2005; 46:3341-3345.
23. Pallavi B, Nagaraj R. Palmitoylated peptides from the cysteine-rich domain of SNAP-23 cause membrane fusion depending on peptide length, position of cysteines, and extent of palmitoylation. J. Biol. Chem. 2003; 278:12737-12744.
24. Creaser SP, Peterson BR. Sensitive and rapid analysis of protein palmitoylation with a synthetic cell-permeable mimic of Src oncoproteins. J. Am. Chem. Soc. 2002; 124:2444-2445.
25. Mayer-Fligge P, Volz J, Kruger U, Sturm E, Gernandt W, Schafer KP, Przybylski M. Synthesis and structural characterization of human-identical lung surfactant SP-C protein. J. Pept. Sci. 1998; 4:355-363.
26. Pachamuthu K, Zhu XM, Schmidt RR. Reversed approach to S-farnesylation and S-palmitoylation: application to an efficient synthesis of the C-terminus of lipidated human N-ras hexapeptide. J. Org. Chem. 2005; 70:3720-3723.
27. Zhu Y, van der Donk WA. Convergent synthesis of peptide conjugates using dehydroalanines for chemoselective ligations. Org. Lett. 2001; 3:1189-1192.
28. Galonic DP, Ide ND, van der Donk WA, Gin DY. Aziridine-2-carboxylic acid-containing peptides: application to solution- and solid-phase convergent site-selective peptide modification. J. Am. Chem. Soc. 2005; 127:7359-7369.
29. Quesnel S, Silvius JR. Cysteine-containing peptide sequences exhibit facile uncatalyzed transacylation and acyl-coa-dependent acylation at the lipid bilayer interface. Biochemistry 1994; 33: 13340-13348.
30. Shahinian S, Silvius JR. Doubly-lipid-modified protein-sequence motifs exhibit long-lived anchorage to lipid bilayer-membranes. Biochemistry 1995; 34:3813-3822.
31. Eisele F, Kuhlmann J, Waldmann H. Synthesis and membrane binding properties of a lipopeptide fragment from influenza virus A hemagglutinin. Chemistry 2002; 8:3362-3376.
32. Kuhn K, Owen DJ, Bader B, Wittinghofer A, Kuhlmann J, Waldmann H. Synthesis of functional Ras lipoproteins and fluorescent derivatives. J. Am. Chem. Soc. 2001; 123:1023-1035.
33. Bader B, Kuhn K, Owen DJ, Waldmann H, Wittinghofer A, Kuhlmann J. Bioorganic synthesis of lipid-modified proteins for the study of signal transduction. Nature 2000; 403:223-226.
34. Volkert M, Uwai K, Tebbe A, Popkirova B, Wagner M, Kuhlmann J, Waldmann H. Synthesis and biological activity of photoactivatable N-Ras peptides and proteins. J. Am. Chem. Soc. 2003; 125:12749-12758.
35. Peters C, Waldmann H. Solid-phase synthesis of peptide esters employing the hydrazide linker. J. Org. Chem. 2003; 68:6053-6055.
36. Palomo JM, Lumbierres M, Waldmann H. Efficient solid-phase lipopeptide synthesis employing the Ellman sulfonamide linker. Angew. Chem. 2006; 118:477-481.
37. Ludolph B, Eisele F, Waldmann H. Solution- and solid-phase synthesis of the polybasic lipid-modified C termini of Rho A and K-Ras 4B. ChemBioChem 2002; 3901-904.
38. Gottlieb D, Grunwald C, Nowak C, Kuhlmann J, Waldmann H. Intein-mediated in vitro synthesis of lipidated Ras proteins. Chem. Commun. 1982; 260-262.
39. Machauer R, Waldmann H. Synthesis of the N-terminal N- myristoylated and S-palmitoylated undetrigintapeptide of endothelial NO-synthase. Angew. Chem. Int. Ed. 2000; 39:1449-1453.
40. Backes BJ, Ellman JA. An alkanesulfonamide “safety-catch” linker for solid-phase synthesis. J. Org. Chem. 1999; 64:2322-2330.
41. Peters C, Wolf A, Wagner M, Kuhlmann J, Waldmann H. The cholesterol membrane anchor of the Hedgehog protein confers stable membrane association to lipid-modified proteins. Proc. Natl. Acad. Sci. U.S.A. 2004; 101:8531-8536.
42. Goody RS, Alexandrov K, Engelhard M. Combining chemical and biological techniques to produce modified proteins. ChemBioChem 2002; 3:399-403.
43. Muir TW, Sondhi D, Cole PA. Expressed protein ligation: a general method for protein engineering. Proc. Natl. Acad. Sci. U.S.A. 1998; 95:6705-6710.
44. Hofmann RM, Muir TW. Recent advances in the application of expressed protein ligation to protein engineering. Curr. Opin. Biotechnol. 2002; 13:297-303.
45. Tolbert TJ, Wong CH. Intein-mediated synthesis of proteins containing carbohydrates and other molecular probes. J. Am. Chem. Soc. 2000; 122:5421-5428.
46. Kochendoerfer GG, Kent SBH. Chemical protein synthesis. Curr. Opin. Chem. Biol. 1999; 3:665-671.
47. Hermanson GT. Bioconjugate Techniques. 1996. Academic Press, Inc., London, UK.
48. Wagner M, Kuhlmann J. Generation and characterization of Ras lipoproteins based on chemical coupling. In: Methods in Molecular Biology, vol. 283: Bioconjugation Protocols: Strategies and Methods. Niemeyer CM, ed. 2004. Humana Press, Totowa, NJ.
49. Muir TW. Semisynthesis of proteins by expressed protein ligation. Annu. Rev. Biochem. 2003; 72:249-289.
50. Durek T, Goody RS, Alexandrov K. In vitro semisynthesis and applications of c-terminally modified rab proteins. In: Methods in Molecular Biology, vol. 283: Bioconjugation Protocols: Strategies and Methods. Niemeyer CM, ed. 2004. Humana Press, Totowa, NJ.
51. Kale TA, Hsieh SJ, Rose MW, Distefano MD. Use of synthetic isoprenoid analogues for understanding protein prenyltransferase mechanism and structure. Curr. Top. Med. Chem. 2003; 3:1043-1074.
52. Musiol HJ, Dong SL, Kaiser M, Bausinger R, Zurnbusch A, Bertsch U, Moroder L. Toward semisynthetic lipoproteins by convergent strategies based on click and Ligation chemistry. ChemBioChem 2005; 6:625-628.
53. Nicolini C, Baranski J, Schlummer S, Palomo JM, Lumbierres M, Kahms M, Kuhlmann J, Sanchez S, Gratton E, Waldmann H, Winter R. Visualizing association of N-Ras in lipid microdomains: influence of domain structure and interfacial adsorption. J. Am. Chem. Soc. 2006; 128:192-201.
54. Reuther G, Tan K-T, Kohler J, Nowak C, Pampel A, Arnold K, Kuhlmann J, Waldmann H, Huster D. Backbone structure of the membrane binding lipid modified C-terminus of the human N-Ras protein. Angew. Chem. Int. Ed. 2006. Submitted.
55. Thutewohl M, Kissau L, Popkirova B, Karaguni IM, Nowak T, Bate M, Kuhlmann J, Muller O, Waldmann H. Solid-phase synthesis and biological evaluation of a pepticinnamin E library. Angew. Chem. Int. Ed. 2002; 41:3616-3620.
56. Deck P, Pendzialek D, Biel M, Wagner M, Popkirova B, Ludolph B, Kragol G, Kuhlmann J, Giannis A, Waldmann H. Development and biological evaluation of acyl protein thioesterase 1 (APT1) inhibitors. Angew. Chem. Int. Ed. 2005; 117:5055-5060.
57. Rocks O, Peyker A, Kahms M, Verveer PJ, Koerner C, Lumbierres M, Kuhlmann J, Waldmann H, Wittinghofer A, Bastiaens PIH. An acylation cycle regulates localization and activity of palmitoylated Ras isoforms. Science 2005; 307:1746-1752.
58. Rak A, Pylypenko O, Durek T, Watzke A, Kushnir S, Brunsveld L, Waldmann H, Goody RS, Alexandrov K. Structure of Rab GDP-dissociation inhibitor in complex with prenylated YPT1 GTPase. Science 2003; 302:646-650.
59. Pylypenko O, Rak A, Durek T, Kushnir S, Dursina BE, Thomae NH, Constantinescu AT, Brunsveld L, Watzke A, Waldmann H, Goody RS, Alexandrov K. Structure of doubly prenylated Ypt1:GDI complex and the mechanism of GDI mediated Rab: membrane interaction. EMBO J. 2006; 25:13-23.
Further Reading
Peptide and lipidated peptide synthesis:
Pegoraro L, Moroder L. In: Houben-Weyl Methods of Organic Chemistry, Synthesis of Peptides and Peptidomimetics, Vol. E 22b. Goodman M, Felix A, Moroder L, Toniolo C, eds. 2003. Thieme, Stuttgart, Germany.
Gelb MH, Brunsveld L, Hrycyna CA, Michaelis S, Tamanoi F, Van Voorhis WC, Waldmann H. Nat. Chem. Biol. 2006; 2:518-528.
Brunsveld L, Kuhlmann J, Alexandrov K, Wittinghofer A, Goody RS, Waldmann H. Angew. Chem. 2006; 118:6774-6798.
Brunsveld L, Kuhlmann J, Waldmann H. Methods 2006; 40:151-165.
Solution phase synthesis of lipidated peptides:
Naider FR, Becker JM. Biopolymers 1997; 43:3-14.
Seitz O, Heinemann I, Mattes A, Waldmann H. Tetrahedron Lett. 2001; 57:2247-2277.
See Also
Coupling Methods: Peptide Synthesis
Lipoproteins, Chemistry of
Peptides, Chemistry of
Peptide Synthesis
Posttranslational Modifications, Chemical