Chemistry for Dummies
Part IV. Chemistry in Everyday Life: Benefits and Problems
Chapter 17. Chemistry in the Home
In This Chapter
· Discovering the chemistry behind cleaners and detergents
· Finding out about the chemistry of cosmetics
· Looking at the chemistry of drugs and medicines
You’ll probably come into direct contact with more chemicals and chemistry in your own home than anyplace else. The kitchen is filled with cleaners, soaps, and detergents, most of which are contained in plastic bottles. The bathroom is filled with medicines, soaps, toothpaste, and cosmetics. My wife is glad to have her own private chemist handy, especially when it’s time to clean the silver or find a solvent to remove an adhesive. And all that doesn’t even cover the myriad chemical reactions that take place while cooking. No wonder consumer chemistry is sometimes called “kitchen chemistry.”
In this chapter, I cover a few topics from the chemistry of consumer products. I show you the chemistry behind soaps, detergents, and cleaners. I talk a little bit about medicines and drugs, and I show you some things about personal care products, permanents, tanning products, and perfumes. I hope that you’ll gain an appreciation for chemistry and what it has done to make your life better and easier. (Note that lots of common chemicals in the home are acids and bases. Chapter 12*s main thing is acids and bases, which makes it nice complementary reading to this chapter.)
Chemistry in the Laundry Room
Have you ever become distracted and forgotten to put the laundry detergent in the washer? Or have you ever been suckered into trying one of those miracle solid ceramic laundry detergent discs? I doubt that the clothes came out very clean. You may have gotten some surface dirt off, but the grease and oil stayed right where it was. The grease and oil stayed on the clothes because “like dissolves like.” Grease and oils are nonpolar materials, and water is a polar substance, so water isn’t going to dissolve the grease and oil. (Chapter 7 gets into detail about this whole polar/nonpolar business, if you’re interested.)
I guess you could dump some gasoline (a nonpolar material) into the washer, but I don’t think that’s a good solution to the problem. Wouldn’t it be wonderful if something existed that could bridge the gap between the nonpolar grease and oil and the polar water? Something does. It’s called a surfactant
Surfactants, which are also called surface active agents, reduce the surface tension of water, allowing it to “wet” nonpolar substances such as grease and oil. Surfactants are able to do this because they have both a nonpolar end and a polar end.
The nonpolar end is called the hydrophobic (water-fearing) end. This end is normally composed of a long hydrocarbon chain. (If you’re feeling ambitious, Chapter 14 covers more than you’ll probably ever want to know about hydrocarbons.) The nonpolar end dissolves in the nonpolar grease and oil.
The other part of the surfactant molecule, the polar end, is called the hydrophilic (water-loving) end. This end is normally an ionic end with a negative charge (anionic), a positive charge (cationic), or both (amphoteric). There are even some surfactants that have no charge (nonionic). (Ions, anions, cations — Chapter 6 explains ’em all.)
A vast majority of the surfactants on the market are anionic surfactants, because they’re cheaper to produce. Figure 17-1 shows a typical anionic surfactant.

Figure 17-1: Atypical anionic surfactant.
When a surfactant is added to water, the hydrophobic end dissolves in the oil and grease, while the hydrophilic end becomes attracted to the polar water molecules. The grease and oil are broken into very tiny droplets called micelles, with the hydrophobic (hydrocarbon) end of the surfactant sticking into the droplet and the hydrophilic end sticking out into the water. This gives the droplet a charge (a negative charge in the case of an anionic surfactant). These charged droplets repel each other and keep the oil and grease droplets from joining together. These micelles remain dispersed and eventually go down the drain with the used wash water.
The two general types of surfactants that are used in the cleaning of clothes are soaps and detergents.
Keep it clean: Soap
Soaps are certainly the oldest and most well-known surfactant for cleaning. The use of soap dates back almost 5,000 years. The specific type of organic reaction involved in the production of soap is a hydrolysis reaction of fats or oils in a basic solution. This reaction is commonly called saponification. The products of this reaction are glycerol and the salt of the fatty acid. Figure 17-2 shows the hydrolysis of tristearin to sodium stearate, a soap. (This is the same soap, or surfactant, shown in Figure 17-1.)
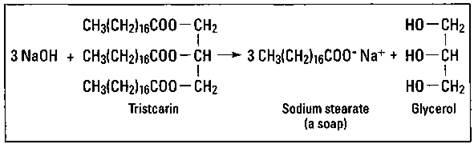
Figure 17-2: Production of a soap by saponification.
Grandma made her soap by taking animal fat, adding it to water and lye (sodium hydroxide, NaOH), and boiling it in a huge iron kettle. The lye came from wood ashes. After cooking for hours, the soap rose to the top. It was then skimmed off and pressed into bars. However, Grandma didn’t know much about reaction stoichiometry. She usually had an excess of lye, so her soap was very alkaline.
Today, soap is made a little differently. The hydrolysis is generally accomplished without the use of lye. Coconut oil, palm oil, and cottonseed oil are used in addition to animal tallow. For bar soaps, an abrasive, such as pumice, is occasionally added to aid in the removal of tough grease and oil from your skin. In addition, perfumes may be added, and air may be mixed with the soap to get it to float.
Soap, however, has a couple of big disadvantages. If soap is used with acidic water, the soap is converted to fatty acids and loses its cleaning ability. And if soap is used with hard water (water containing calcium, magnesium, or iron ions), a greasy insoluble precipitate (solid) forms. This greasy deposit is commonly called bathtub ring. And it’s a bummer. Not only does the deposit form in your bathtub, but it also appears on your clothes, dishes, and so on. A couple of ways are available to avoid this deposit. You can use a whole-house water softener (see “Make it soft: Water softeners,” later in this chapter), or you can buy a synthetic soap that doesn’t precipitate with hard-water ions. These synthetic soaps are called detergents.
Get rid of that bathtub ring: Determents
Detergents have the same basic structure as the soap in Figure 17-1. Their hydrophobic end — composed of a long nonpolar hydrocarbon chain that dissolves in the grease and oil — is the same, but their hydrophilic (ionic) end is different. Instead of having a carboxylate (-COO-), the hydrophilic end may have a sulfate (-O-SO3-), a hydroxyl (OH), or some other polar group that doesn’t precipitate with hard water.
Laundry detergents contain a number of other compounds in addition to the detergent surfactant. The compounds in laundry detergents are
ü Builders: These compounds increase the surfactant’s efficiency by softening the water (removing the hard water ions) and making it alkaline. The builder that was used in early laundry detergents was sodium tripolyphosphate. It was cheap and safe. However, it was also an excellent nutrient for water plants and caused an increase in the growth of algae in lakes and streams, choking out fish and other aquatic life. States began banning the use of phosphates in detergents in order to control this problem. Sodium carbonate and zeolites (complex aluminosilicates — compounds of aluminum, oxygen, and silicon) have been used as replacements for the polyphosphates, but both are less than ideal. There really hasn’t been an effective, cheap, and nontoxic replacement for the polyphosphate builders. This is an area of research that’s still quite active.
ü Fillers: Compounds such as sodium sulfate (Na2SO4) are added to give the detergent bulk and to keep it free-flowing.
ü Enzymes: These biological catalysts are sometimes added to help remove protein-based stains such as blood and grass.
ü Sodium perborate: NaBO3 is sometimes added as a solid bleach to help remove stains. It works by generating hydrogen peroxide in water. It’s much more gentle on textiles than chlorine bleach. However, it’s most effective in hot water, which can present a problem for those of us who like to wash in cold water.
ü Suspension agents: These compounds are added to help keep the dirt in solution in the wash water so that it doesn’t redeposit itself on another portion of the clothes.
ü Corrosion inhibitors: These compounds coat washer parts to help prevent rust.
ü Optical brighteners: These compounds are used to make white clothes appear extra clean and bright. These very complex organic compounds deposit themselves as a thin coating on the clothes. They absorb ultraviolet light and re-emit it as a blue light in the visible part of the spectrum. This process is shown in Figure 17-3.
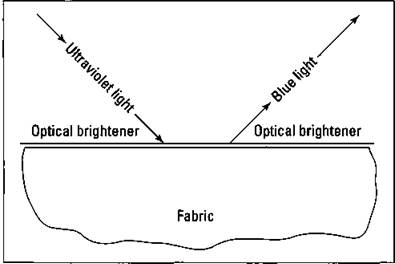
Figure 17-3: Optical brighteners.
Coloring agents and perfumes are added to laundry detergent, as well. I bet you didn’t know that washing clothes was so complex.
Make it soft: Water softeners
Using synthetic detergents is one way to combat the problem of hard water and bathtub ring. Another way is to simply remove the cations responsible for the hard water before they reach the house. You can accomplish this feat through a home water softener (see Figure 17-4).
A water softener consists of a large tank containing an ion-exchange resin. The resin is charged when a concentrated sodium chloride solution runs through it. The sodium ions are held to the polymer material of the resin. The hard water passes through the polymer, and the calcium, magnesium, and iron ions are exchanged for the sodium ions on the resin (that’s where the term ion-exchange resin comes from). The softened water contains sodium ions, but the hard-water ions remain in the resin. After a while, the resin must be recharged with more sodium chloride from the reservoir. The wastewater that contains the Ca2+, Mg2+, and Fe2+ is drained from the resin tank.
What's that froth in the lake?
The original synthetic detergents weren't capable of being broken down by bacteria and other natural forces. In other words, they weren't biodegradable. These detergents accumulated in lakes and streams and caused a thick coating of suds. They were quickly reformulated to solve the problem.
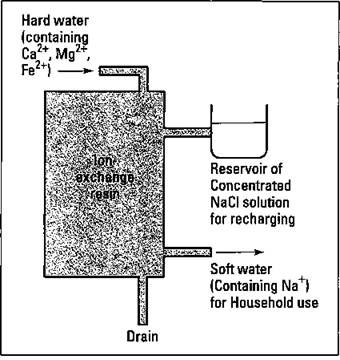
Figure 17-4: A whole-house water softener.
WARNING! If you limit your sodium intake because of high blood pressure, you should avoid drinking softened water because it has a high sodium ion concentration.
Make it whiter: Bleach
Bleaches use redox reactions to remove color from material (see Chapter 9 for a discussion of redox reactions). Most bleaches are oxidizing agents. The most common bleach used in the home is a 5 percent solution of sodium hypochlorite. This type of bleach is produced by bubbling chlorine gas through a sodium hydroxide solution:
![]()
The chlorine released by hypochlorite bleaches can damage fabrics. Also, these types of bleaches don’t work very well on polyester fabrics.
Bleaches containing sodium perborate have been introduced to the market, and they’re gentler on fabrics. This type of bleach generates hydrogen peroxide, which in turn decomposes with oxygen gas as one of the products:
![]()
Chemistry in the Kitchen
You can take a peek under the kitchen sink and see countless products that are made with chemicals (and stored in plastic bottles that are made through chemistry).
Clean it all: Multipurpose cleaners
Most multipurpose cleaners are composed of some surfactant and disinfectant. Ammonia is commonly used because of its ability to react with grease and because it leaves no residue. Pine oil, a solution of compounds called terpenes, is used for its pleasant odor, its ability to dissolve grease, and its antibacterial nature.
WARNING! Be careful when mixing household cleaning products — especially bleach with ammonia or muratic acid (HCl). This solution generates toxic gases that can be quite dangerous.
Wash those pots: Dishwashing products
Dishwashing detergent is much simpler than laundry detergent. It has some surfactant (normally a nonionic one), a little colorant, and something to make your hands feel soft.
Dishwashing detergent is not nearly as alkaline as laundry detergent. However, automatic dishwasher detergents are highly alkaline and contain only a little surfactant. They use the high pH to saponify the fats (like the process used to make soap) and a high water temperature as well as agitation to clean the dishes. They’re composed mostly of sodium metasilicate (Na2SiO3), for its alkalinity; sodium tripolyphosphate (Na5P3O10), which acts as a detergent; and a little chlorine bleach.
Chemistry in the Bathroom
A lot of chemistry goes on in the bathroom. There are all those skin and hair care products, as well as products to make you look good and smell good and even taste good.
Detergent for the mouth: Toothpaste
Walk down any toothpaste aisle, and you’ll see a wide variety of toothpastes with different colors, flavors, and so on. Although they may look different, they all contain the same basic ingredients. The two primary ingredients are surfactant (detergent) and abrasive. The abrasive is for scraping the film off the teeth without damaging the teeth themselves. Common abrasives are chalk (CaCO3), titanium dioxide (TiO2), and calcium hydrogen phosphate (CaHPO4). Other ingredients are added to give the toothpaste color, flavoring, and so on. Table 17-1 gives the general formula for toothpaste. The percentages and specific chemical compounds may vary from toothpaste to toothpaste.
Table 17-1. Typical Formulation for Toothpaste
|
Function |
Possible Ingredient Percentage |
|
|
Solvent and filler |
Water |
30-40% |
|
Detergent |
Sodium lauryl sulfate, soap |
4% |
|
Abrasive |
Calcium carbonate, calcium hydrogen phosphate, titanium dioxide, sodium metaphosphate, silicia, aluminia |
30-50% |
|
Sweetener |
Glycerine, saccharin, sorbitol |
15-20% |
|
Thickener |
Gum cellulose, carrageenan |
1% |
|
Fluoride |
Stannous or sodium fluoride |
1% |
|
Flavoring |
Oil of wintergreen, peppermint, strawberry, lime, and so on |
1% |
The addition of stannous or sodium fluoride is effective in the prevention of dented cavities, because the fluoride ion actually becomes part of the tooth enamel, making the enamel stronger and more resistant to the attack of acids.
Phew! Deodorants and antiperspirants
Sweating helps your body regulate its internal temperature. Sweat contains amines, low molecular weight fatty acids, and proteins, in addition to sodium chloride and other inorganic compounds. Some of these organic compounds have a disagreeable odor. Bacterial action can certainly make the odor worse. Deodorants and antiperspirants can be used to control the socially unacceptable odor. (Quite a professional way to discuss stinky B.O., eh?)
Deodorants contain fragrances to cover up the odor and an antibacterial agent to destroy the odor-causing bacteria. They may also contain substances such as zinc peroxide that oxidize the amines and fatty acids to less odorous compounds.
Antiperspirants inhibit or stop perspiration. They act as an astringent, constricting the sweat gland ducts. The most commonly used antiperspirants are compounds of aluminum — aluminum chlorohydrates (Al2(OH)5Cl, Al2(OH)4Cl2, and so on), hydrated aluminum chloride (AlCl36H2O), and others.
Skin care chemistry: Keepiny it soft and pretty
Beeswax. Whale wax. Borax. You may be surprised at what’s in some of that stuff you put on your skin.
Creams and lotions
The skin is a complex organ composed primarily of protein and naturally occurring macromolecules (polymers — see Chapter 16). Healthy skin contains about 10 percent moisture. Creams and lotions work to soften and moisturize the skin.
Emollients are skin softeners. Petroleum jelly (mixture of alkanes, with 20-plus carbons, isolated from crude oil), lanolin (mixture of esters isolated from sheep wool fat), and coco butter (mixture of esters isolated from the cacao bean) are excellent skin softeners.
Skin creams are normally made of oil-in-water or water-in-oil emulsions. An emulsion is a colloidal dispersion of one liquid in another (see Chapter 11 for a discussion of colloids). It tries to soften and moisturize the skin at the same time. Cold creams are used in the removal of makeup and as moisturizers, while vanishing creams make the skin appear younger by filling in wrinkles. Typical formulations for cold cream and vanishing cream are
|
Cold Cream Formulation |
Vanishing Cream Formulation |
|
20-50% water |
70% water |
|
30-60% mineral oil |
10% glycerin |
|
12-15% beeswax |
20% stearic acid/sodium stearate |
|
|
5-15% lanolin or whale wax |
|
|
1% borax |
|
|
trace of perfume |
Body and face powders
Body and face powders are used to dry and smooth the skin. The main ingredient in both types of powder is talc (Mg3(Si2O5)2(OH)2), a mineral that absorbs both oil and water. Astringents are added to reduce sweating, and binders are added to help the powders stick to the skin better. Face powders often contain dyes to give color to the skin. Table 17-2 shows a typical formulation for body powder, and Table 17-3 shows a typical formulation for face powder.
Table 17-2. Typical Formulation for Body Powder
|
Ingredient |
Function |
Percentage |
|
|
Talc |
Absorbent, bulk |
50-60% |
|
|
Chalk (CaCO3) |
Absorbent |
10-15% |
|
|
Zinc oxide (ZnO) |
Astringent |
15-25% |
|
|
Zinc stearate |
Binder |
5-10% |
|
|
Perfume, dye |
Odor, color |
trace |
|
Table 17-3. Typical Formulation for Face Powder
|
Ingredient |
Function |
Percentage |
|
Talc |
Absorbent, bulk |
60-70% |
|
Zinc oxide |
Astringent |
10-15% |
|
Kaolin (Al2SiO5) |
Absorbent |
10-15% |
|
Magnesium and zinc stearates |
Texture |
5-15% |
|
Cetyl alcohol |
Binder |
1% |
|
Mineral oil |
Emollient |
2% |
|
Lanolin, perfume, dyes |
Softening, odor, color |
2% |
Making up those eyes
Eye shadow and mascara are composed primarily of emollients, lanolin, beeswax, and colorants. Mascara darkens the eyelashes, making them appear longer. Typical formulations for eye shadow and mascara are
|
Eye Shadow |
Mascara |
|
55-60% petroleum jelly |
45-50% soap |
|
5-15% fats and waxes |
35-40% wax and paraffin |
|
5-10% lanolin |
5-10% lanolin |
|
15-25% zinc oxide |
1-5% dyes |
|
1-5% dyes |
|
Kissable tips: Lipstick
Lipstick keeps the lips soft and protects them from drying out, while adding a desirable color. It’s composed mostly of wax and oil. These ingredients must be balanced carefully so that the lipstick goes on easily without running and comes off easily, but not too easily, when the wearer is ready to remove it. The color normally comes from a precipitate (solid) of some metal ion with an organic dye. This is commonly called a lake. The metal ion tends to intensify the color of the dye. A typical lipstick formulation is shown in Table 17-4.
Table 17-4. A Typical Lipstick Formulation
|
Ingredient |
Function |
Percentage |
|
Castor oil, mineral oil, fats |
Dye solvent |
40-50% |
|
Lanolin |
Emollient |
20-30% |
|
Carnauba wax or beeswax |
Stiffener |
15-25% |
|
Dye |
Color |
5-10% |
|
Perfume and flavoring |
Odor and taste |
trace |
Beautiful nails: Nail polish
Nail polish is a synthetic lacquer that owes its flexibility to a polymer and a plasticizer (a liquid mixed with plastics to soften them). The polymer is normally nitrocellulose. The solvents used in the polish are acetone and ethyl acetate, the same substances used for nail polish removers.
Smelling GOOD! Perfumes, colognes, and aftershaves
The major difference between perfume, cologne, and aftershave is the amount of fragrance used. Perfumes are commonly composed of 10 to 25 percent fragrance, while colognes use 1 to 3 percent, and aftershaves use less than 1 percent. These fragrances are usually organic esters, alcohols, ketones, and aldehydes. Perfumes also contain fixatives, compounds that help keep the fragrances from evaporating too rapidly.
Interestingly, several fixatives have disagreeable odors or histories themselves: Civetone comes from the glands of the skunk-like civet cat, ambergris is sperm-whale vomit, and indole is isolated from feces. I don’t think I’ll comment on this.
Perfumes are usually mixtures of notes, fragrances with similar aromas but different volatilities (the ease with which a substance is converted into a gas). The most volatile is called the top note. It’s what you initially smell. The middle note is the most noticeable smell, while the end note fragrances are responsible for the lingering odor of the perfume. Figure 17-5 shows the chemical structure of several of the fragrances commonly used in perfumes.
Don’t you think it’s neat being able to see an odor?
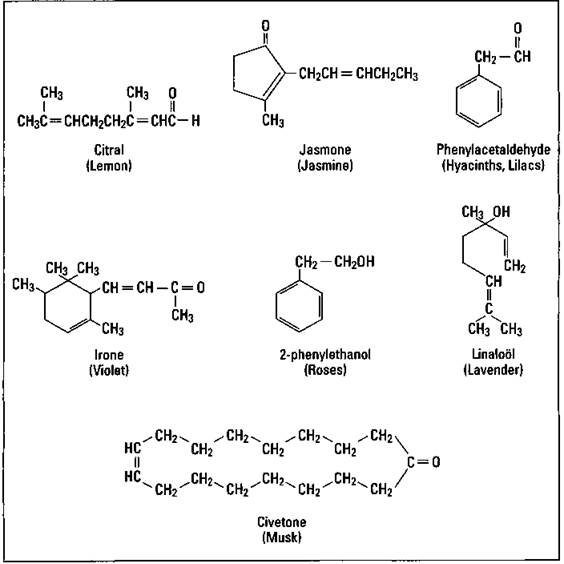
Figure 17-5: Perfume fragrances.
Suntan lotion and sunscreen: Brown is beautiful
A suntan is nature’s way of protecting our bodies against the harmful UV rays of the sun. The UV spectrum is composed of two regions: the UV-A region and the UV-B region. The UV-A region has slightly longer wavelengths and tends to produce a tan rather than a burn. UV-B radiation is what’s responsible for those quick sunburns most of us are familiar with. Repeated exposure to both of these harmful UV rays, especially UV-B rays, is related to an increase in the occurrences of skin cancers, such as melanoma.
Suntan lotions and sunscreens protect the skin by partially or totally blocking the sun’s radiation in the UV range, allowing you to be exposed to the sun for longer periods of time without burning. Some suntan lotions and sunscreens block both the UV-A and UV-B regions. Other types selectively block the UV-B regions, allowing the UV-A rays through, which gives the body a chance to produce melanin, a dark pigment that acts as a natural shield against the sun’s UV rays, and that desirable brown skin tone.
These products are given a Sun Protection Factor (SPF) rating. The SPF value is a ratio of the amount of time required to tan (or burn) with versus without the product. An SPF value of 10, for example, indicates that when using the product, you can be exposed to the sun 10 times as long without burning.
There’s some debate about whether SPF values above 15 are any more effective than the value 15, because few tanning products effectively block the UV-A radiation. The FDA is currently examining tanning products carefully.
A number of chemical substances are effective at blocking UV radiation. An opaque cream of zinc oxide and titanium dioxide is the most effective type of sunscreen. In addition, para-aminobenzoic acid (PABA), benzophenone, and cinnamates are commonly used to block UV radiation. Recently, there has been a move away from the use of PABA, though. It’s somewhat toxic, and a significant number of individuals are allergic to it.
Figure 17-6 shows the structures of several compounds used in suntan and sunscreen products. The dihydroxyacetone shown in the figure produces a tan without exposure to the sun. It reacts with the skin to produce a brown pigment.
Clean it, color it, curl it: Hair care chemistry
Hair is composed of a protein called keratin. The protein chains in the hair strand are connected to each other by what’s called a disulfide bond, a sulfur- to-sulfur bond from the cystine (an amino acid component of hair) on one protein chain to another cystine on another protein chain.
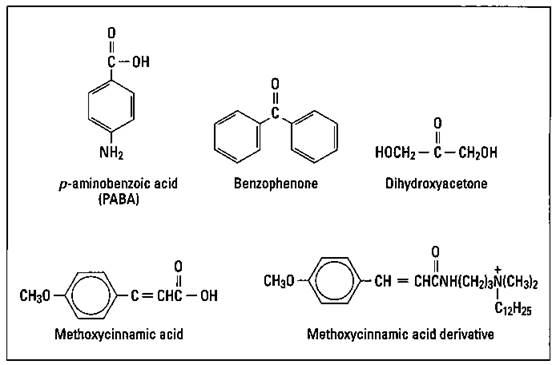
Figure 17-6: Tanning and sunscreen products.
Figure 17-7 shows a portion of hair and disulfide crosslinks joining two protein chains. These crosslinks give hair its strength. (I say more about this disulfide bond in “Permanents — that aren’t,” a little later in this section.)
Shampoos: Determents for the hair
Modern shampoos are simple surfactants, such as sodium lauryl sulfate and sodium dodecyl sulfate. Shampoo contains other ingredients, however, that react with the metal ions in hard water to help prevent the soap from precipitating with these metal ions (in other words, to help prevent insoluble precipitates — solids, deposits, bathtub ring — from forming in your hair).
Other ingredients give a pleasant odor, replace some of the natural lubricants in the hair (conditioners), and adjust the hair’s pH. (Hair and skin are slightly acidic. A very alkaline, or basic, shampoo will damage hair, so the pH is commonly adjusted to the 5 to 8 pH range. A higher pH may also make the scales on the hair cuticles fan out and reflect the light poorly, making the hair look flat and dull.) A protein is sometimes added to the shampoo to help glue damaged split-ends together. Colorants and preservatives are also commonly added, too.
Color that hair!
Hair contains two pigments — melanin and phaeomelanin. Melanin has a dark-brown color, and phaeomelanin has a reddish-brown color. The natural color of the hair is determined by the relative amounts of these pigments.
Red heads have much less melanin; brunettes have much more. Blondes have very little of either.
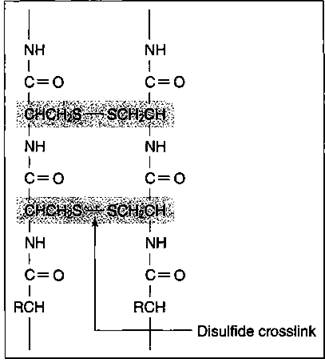
Figure 17-7: Disulfide bond in hair.
You can bleach hair by using hydrogen peroxide to oxidize these colored pigments to their colorless forms. However, bleached hair becomes weaker and more brittle, because the hair protein is broken down into lower molecular weight compounds. Perborate compounds, which tend to be more expensive than bleach, and chlorine-based bleaches are also sometimes used to bleach hair.
You can change the color of your hair temporarily by using dyes that simply coat the hair strands. These compounds are composed of complex organic molecules. They’re too large to penetrate the hair strand, so they simply accumulate on the surface. You can add semi-permanent color by using dyes with smaller molecules that can penetrate into the hair. These dyes frequently contain complexes of chromium or cobalt. The dyes withstand repeated washing, but because the molecules contained in the dye were small enough to penetrate the hair initially, they eventually migrate out.
Permanent dyes are actually formed inside the hair. Small molecules are forced into the hair and then oxidized, normally by hydrogen peroxide, into colored complexes that are too large to migrate out of the hair. The color then becomes permanent on the portion of the hair that was treated. To maintain the color, you have to repeat the process as new hair grows out. This maintenance program keeps hairdressers in business.
Another type of hair coloring is made to change color gradually, over a period of weeks, so that the change goes unnoticed (fat chance!). A solution of lead acetate is applied to the hair. The lead ions react with the sulfur atoms in the hair protein, forming lead (II) sulfide (PbS), which is black and very insoluble. Instead of losing its color in the sunlight like other dyes, PbS-treated hair actually darkens.
Take it off, take it all off! Depilatories
Depilatories remove hair by chemical reaction. They contain a substance, usually sodium sulfide, calcium sulfide, or calcium thioglycolate, that disrupts the disulfide linkages in the hair and dissolves it. The formulations commonly contain a base such as calcium hydroxide to raise the pH and enhance the action of the depilatory. A detergent and a skin conditioner such as mineral oil are also generally added to depilatories.
Permanents — that aren’t
Disulfide bonds are responsible for the shape of your hair, whether it’s straight or curly. In order to affect the shape of hair, those disulfide bonds must be broken and reformed into a new orientation. Suppose, for example, that you want to make your straight hair curly, so you go to the beauty parlor for a permanent. The hairdresser initially treats your hair with some reducing agent that breaks the disulfide bonds; thioglycolic acid (HS-CHrCOOH) is commonly used. Then the hairdresser changes the orientation of the protein chains of the hair by using curlers. Finally, the hairdresser treats your hair with an oxidizing agent such as hydrogen peroxide to reform the disulfide bonds in their new locations. Water-soluble polymers are used to thicken the solutions, ammonia is used to adjust the pH to a basic level, and a conditioner is used to complete the formulation. Figure 17-8 shows this process.
Hair is straightened in exactly the same fashion, except it’s stretched straight instead of curled. Obviously, as new hair grows in, you need to repeat the process.
I guess permanent refers to the fact that trips to the beauty parlor become a permanent part of your life.
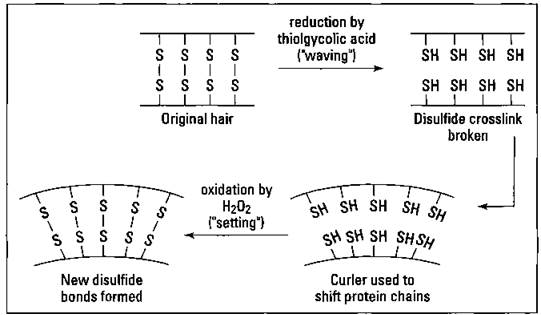
Figure 17-8: The process of getting a perm.
Chemistry in the Medicine Cabinet
Okay, take a quick peek in the medicine cabinet. There are a lot of drugs and medicines inside there. I could spend pages and pages talking about the chemistry of their reactions and interactions, but I’m just going to say a few brief words about a couple of them.
The aspirin story
As early as the fifth century b.c., it was known that chewing willow bark could relieve pain. But it wasn’t until 1860 that the chemical compound responsible for the analgesic effect, salicylic acid, was isolated. It had a very sour taste and caused irritation of the stomach. In 1875, chemists created sodium salicylate. It caused stomach irritation, but it was less bitter them the salicylic acid. Finally, in 1899, the German Bayer Company began marketing acetylsalicylic acid, made by reacting salicylic acid with acetic anhydride, under the trade name of aspirin. Figure 17-9 traces the history of aspirin.
Aspirin is the most widely used drug in the world. More than 55 billion aspirin tablets are sold annually in the United States.
Minoxidil and Viagra
Science proceeds by hard work, training, intuition, hunches, and luck. That luck is sometimes called serendipity; which is another name for an accidental discovery. Or, as I like to say, “finding something you didn’t know you were looking for.” Chapter 20 tells the stories of ten serendipitous discoveries. But because I’m in the medicine cabinet anyway, I may as well mention a couple of serendipitous discoveries right now.
Male pattern baldness affects many millions of men and women in the world. Minoxidil, the current over-the-counter treatment for baldness, was discovered quite by accident. It was being used as an oral treatment for high blood pressure, when patients reported hair growth. Now it’s usually applied topically instead of orally.
The much-publicized properties of Viagra were discovered in much the same fashion. It was also being used as a treatment for high blood pressure, as well as angina (heart pain), when its side effect was reported. In fact, it has been said that male patients refused to return the unused portion of their medications during clinical trials.
Both serendipitous discoveries have spawned multimillion-dollar industries and made countless men and women very happy. They’re growing industries, for sure.
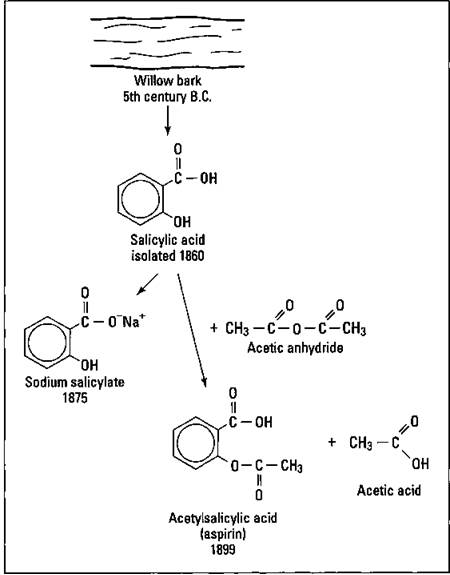
Figure 17-9: The aspirin story.