Chemistry for Dummies
Part I. Basic Concepts of Chemistry
Chapter 2. Matter and Energy
In This Chapter
· Understanding the states of matter and their changes
· Differentiating between pure substances and mixtures
· Finding out about the metric system
· Examining the properties of chemical substances
· Discovering the different types of energy
· Measuring the energy in chemical bonds
Walk into a room and turn on the light. Look around — what do you see? There might be a table, some chairs, a lamp, a computer humming away. But really all you see is matter and energy. There are many kinds of matter and many kinds of energy, but when all is said and done, you’re left with these two things. Scientists used to believe that these two were separate and distinct, but now they realize that matter and energy are linked. In an atomic bomb or nuclear reactor, matter is converted into energy. Perhaps someday the science fiction of Star Trek will become a reality and converting the human body into energy and back in a transporter will be commonplace. But in the meantime, I’ll stick to the basics of matter and energy.
In this chapter, I cover the two basic components of the universe — matter and energy. I examine the different states of matter and what happens when matter goes from one state to another. I show you how the metric system is used to make matter and energy measurements, and I examine the different types of energy and see how energy is measured.
States of Matter: Macroscopic and Microscopic Views
Look around you. All the stuff you see — your chair, the water you’re drinking, the paper this book is printed on — is matter. Matter is the material part of the universe. It’s anything that has mass and occupies space. (Later in this chapter, I introduce you to energy, the other part of the universe.) Matter can exist in one of three states: solid, liquid, and gas.
Solids
At the macroscopic level, the level at which we directly observe with our senses, a solid has a definite shape and occupies a definite volume. Think of an ice cube in a glass — it’s a solid. You can easily weigh the ice cube and measure its volume. At the microscopic level (where items are so small that people can’t directly observe them), the particles that make up the ice are very close together and aren’t moving around very much (see Figure 2-1a).
The reason the particles that make up the ice (also known as water molecules) are close together and have little movement is because, in many solids, the particles are pulled into a rigid, organized structure of repeating patterns called a crystal lattice. The particles that are contained in the crystal lattice are still moving, but barely — it’s more of a slight vibration. Depending on the particles, this crystal lattice may be of different shapes.
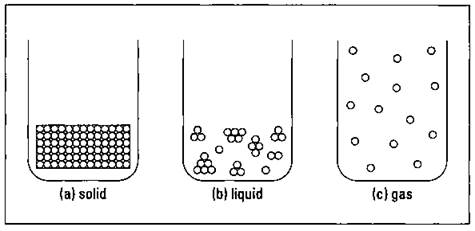
Figure 2-1: Solid, liquid, and gaseous states of matter.
Liquids
When an ice cube melts, it becomes a liquid. Unlike solids, liquids have no definite shape, but they do have a definite volume, just like solids do. For example, a cup of water in a tall skinny glass has a different shape than a cup of water in a pie pan, but in both cases, the volume of water is the same — one cup. Why? The particles in liquids are much farther apart than the particles in solids, and they’re also moving around much more (see Figure 2-lb.). Even though the particles are farther apart in liquids than in solids, some particles in liquids may still be near each other, clumped together in small groups.
Because the particles are farther apart in liquids, the attractive forces among them aren’t as strong as they are in solids — which is why liquids don’t have a definite shape. However, these attractive forces are strong enough to keep the substance confined in one large mass — a liquid — instead of going all over the place.
Gases
If you heat water, you can convert it to steam, the gaseous form of water. A gas has no definite shape and no definite volume. In a gas, particles are much farther apart than they are in solids or liquids (see Figure 2-1c), and they’re moving relatively independent of each other. Because of the distance between the particles and the independent motion of each of them, the gas expands to fill the area that contains it (and thus it has no definite shape).
Ice in Alaska, Water in Texas: Matter Changes States
When a substance goes from one state of matter to another, we call the process a change of state. Some rather interesting things occur during this process.
I’m melting away! Oh, What a World!
Imagine taking a big chunk of ice out of your freezer and putting it into a large pot on your stove. If you measure the temperature of that chunk of ice, you may find it to be -5° Celsius or so. If you take temperature readings while heating the ice, you find that the temperature of the ice begins to rise as the heat from the stove causes the ice particles to begin vibrating faster and faster in the crystal lattice. After a while, some of the particles move so fast that they break free of the lattice, and the crystal lattice (which keeps a solid solid) eventually breaks apart. The solid begins to go from a solid state to a liquid state — a process called melting. The temperature at which melting occurs is called the melting point (mp) of the substance. The melting point for ice is 32° Fahrenheit, or 0° Celsius.
If you watch the temperature of ice as it melts, you see that the temperature remains steady at 0°C until all the ice has melted. During changes of state (phase changes), the temperature remains constant even though the liquid contains more energy than the ice (because the particles in liquids move faster than the particles in solids, as mentioned in the previous section).
Boiling point
If you heat a pot of cool water (or if you continue to heat the pot of now-melted ice cubes mentioned in the preceding section), the temperature of the water rises and the particles move faster and faster as they absorb the heat. The temperature rises until the water reaches the next change of state — boiling. As the particles move faster and faster as they heat up, they begin to break the attractive forces between each other and move freely as steam — a gas. The process by which a substance moves from the liquid state to the gaseous state is called boiling. The temperature at which a liquid begins to boil is called the boiling point (bp). The bp is dependent on atmospheric pressure, but for water at sea level, it’s 212°F, or 100°C. The temperature of the boiling water will remain constant until all the water has been converted to steam.
You can have both water and steam at 100°C. They will have the same temperature, but the steam will have a lot more energy (because the particles move independently and pretty quickly). Because steam has more energy, steam bums are normally a lot more serious than boiling water burns — much more energy is transferred to your skin. I was reminded of this one morning while trying to iron a wrinkle out of a shirt that I was still wearing. My skin and I can attest — steam contains a lot of energy!
I can summarize the process of water changing from a solid to a liquid in this way:
ice→water→steam
Because the basic particle in ice, water, and steam is the water molecule (written as H2O), the saune process can also be shown as
![]()
Here the (s) stands for solid, the (l) stands for liquid, and the (g) stands for gas. This second depiction is much better, because unlike H2O, most chemical substances don’t have different names for the solid, liquid, and gas forms.
Freezing point: The miracle of ice cubes
If you cool a gaseous substance, you can watch the phase changes that occur. The phase changes are
ü Condensation — going from a gas to a liquid
ü Freezing — going from a liquid to a solid
The gas particles have a high amount of energy, but as they’re cooled, that energy is reduced. The attractive forces now have a chance to draw the particles closer together, forming a liquid. This process is called condensation. The particles are now in clumps (as is characteristic of particles in a liquid state), but as more energy is removed by cooling, the particles start to align themselves, and a solid is formed. This is known as freezing. The temperature at which this occurs is called the freezing point (fp) of the substance.
REMEMBER. The freezing point is the same as the melting point — it’s the point at which the liquid is able to become a gas or solid.
I can represent water changing states from a gas to a solid like this:
![]()
Sublimate this!
Most substances go through the logical progression from solid to liquid to gas as they’re heated — or vice versa as they’re cooled. But a few substances go directly from the solid to the gaseous state without ever becoming a liquid. Scientists call this process sublimation. Dry ice — solid carbon dioxide, written as CO2(s) — is the classic example of sublimation. You can see dry ice particles becoming smaller as the solid begins to turn into a gas, but no liquid is formed during this phase change. (If you’ve seen dry ice, then you remember that a white cloud usually accompanies it — magicians and theater productions often use dry ice for a cloudy or foggy effect. The white cloud you normally see isn’t the carbon dioxide gas — the gas itself is colorless. The white cloud is the condensation of the water vapor in the air due to the cold of the dry ice.)
The process of sublimation is represented as
![]()
In addition to dry ice, mothballs and certain solid air fresheners also go through the process of sublimation. The reverse of sublimation is deposition — going directly from a gaseous state to a solid state.
Pure Substances and Mixtures
One of the basic processes in science is classification. As discussed in the preceding section, chemists cam classify matter as solid, liquid, or gas. But there are other ways to classify matter, as well. In this section, I discuss how all matter can be classified as either a pure substance or a mixture (see Figure 2-2).
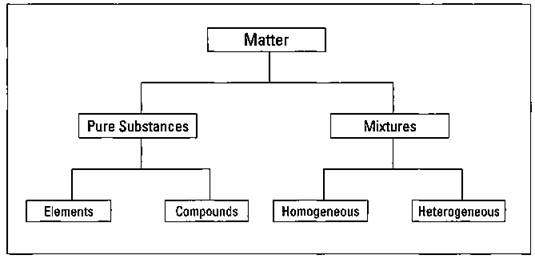
Figure 2-2: Classification of matter.
Pure substances
A pure substance has a definite and constant composition or make-up — like salt or sugar.
A pure substance can be either an element or a compound, but the composition of a pure substance doesn’t vary.
Elementary, my dear reader
An element is composed of a single kind of atom. An atom is the smallest particle of an element that still has all the properties of the element. Here’s an example: Gold is an element. If you slice and slice a chunk of gold until only one tiny particle is left that can’t be chopped any more without losing the properties that make gold gold, then you’ve got an atom.
The atoms in an element all have the same number of protons. Protons are subatomic particles — particles of an atom. There are three major subatomic particles, which Chapter 3 covers in great, gory detail.
The important thing to remember right now is that elements are the building blocks of matter. And they’re represented in a strange table you may have seen at one time or another — the periodic table. (If you haven’t seen such a table before, it’s just a list of elements. Chapter 3 contains one if you want to take a peek.)
Compounding the problem
A compound is composed of two or more elements in a specific ratio. For example, water (H2O) is a compound made up of two elements, hydrogen (H) and oxygen (O). These elements are combined in a very specific way — in a ratio of two hydrogen atoms to one oxygen atom (hence H2O). A lot of compounds contain hydrogen and oxygen, but only one has that special 2 to 1 ratio we call water. Even though water is made up of hydrogen and oxygen, the compound water has physical and chemical properties different from both hydrogen and oxygen — water’s properties are a unique combination of the two elements.
Chemists can’t easily separate the components of a compound: They have to resort to some type of chemical reaction.
Throwing mixtures into the mix
Mixtures are physical combinations of pure substances that have no definite or constant composition — the composition of a mixture varies according to who prepares the mixture. Suppose I asked two people to prepare me a margarita (a delightful mixture). Unless these two people used exactly the same recipe, these mixtures would vary somewhat in their relative amounts of tequila, triple sec, and so on. They would have produced two slightly different mixtures. However, each component of a mixture (that is, each pure substance that makes up the mixture — in the drink example, each ingredient) retains its own set of physical and chemical characteristics. Because of this, it’s relatively easy to separate the various substances in a mixture.
Although chemists have a difficult time separating compounds into their specific elements, the different parts of a mixture can be easily separated by physical means, such as filtration. For example, suppose you have a mixture of salt and sand, and you want to purify the sand by removing the salt. You can do this by adding water, dissolving the salt, and then filtering the mixture. You then end up with pure sand.
Mixtures can be either homogeneous or heterogeneous.
Homogeneous mixtures, sometimes called solutions, are relatively uniform in composition; every portion of the mixture is like every other portion. If you dissolve sugar in water and mix it really well, your mixture is basically the same no matter where you sample it.
But if you put some sugar in a jar, add some sand, and then give the jar a couple of shakes, your mixture doesn’t have the same composition throughout the jar. Because the sand is heavier, there’s probably more sand at the bottom of the jar and more sugar at the top. In this case, you have a heterogeneous mixture, a mixture whose composition varies from position to position within the sample.
Measuring Matter
Scientists are often called on to make measurements, which may include such things as mass (weight), volume, and temperature. If each nation had its own measurement system, communication among scientists would be tremendously hampered, so a worldwide measurement system has been adopted to ensure that scientists can speak the same language.
The SI system
The SI system (from the French Systeme International) is a worldwide measurement system based on the older metric system that most of us learned in school. There are minor differences between the SI and metric systems, but, for purposes of this book, they’re interchangeable.
SI is a decimal system with basic units for things like mass, length, and volume, and prefixes that modify the basic units. For example, the prefix kilo- (k) means 1,000. So a kilogram (kg) is 1,000 grams and a kilometer (km) is 1,000 meters. Two other very useful SI prefixes are centi- (c) and milli- (m), which mean 0.01 and 0.001, respectively. So a milligram (mg) is 0.001 grams — or you can say that there are 1,000 milligrams in a gram. (Check out Appendix A for the most useful SI prefixes.)
SI/Enytish conversions
Many years ago, there was a movement in the United States to convert to the metric system. But, alas, Americans are still buying their potatoes by the pound and their gasoline by the gallon. Don’t worry about it. Most professional chemists I know use both the U.S. and SI systems without any trouble. It’s necessary to make conversions when using two systems, but I show you how to do that right here.
The basic unit of length in the SI system is the meter (m). A meter is a little longer than a yard; there are 1.094 yards in a meter, to be exact. But that’s not a really useful conversion. The most useful SI/English conversion for length is
2.54 centimeters = 1 inch
The basic unit of mass in the SI system for chemists is the gram (g). And the most useful conversion for mass is
454 grams = 1 pound
The basic unit for volume in the SI system is the liter (L). The most useful conversion is
0.946 liter = 1 quart
TIP. By using the preceding conversions and the unit conversion method I describe in Appendix C, you’ll be able to handle most SI/English conversions you need to do.
For example, suppose that you have a 5-pound sack of potatoes and you want to know its weight in kilograms. Write down 5 pounds (lbs) as a fraction by placing it over 1.
![]()
Because you need to cancel the unit lbs in the numerator, you must find a relationship between lbs and something else — and then express that something else with lbs in the denominator. You know the relationship between pounds and grams, so you can use that.
![]()
Now simply convert from grams to kilograms in the same way.
![]()
Nice Properties You've Got There
When chemists study chemical substances, they examine two types of properties:
ü Chemical properties: These properties enable a substance to change into a brand-new substance, and they describe how a substance reacts with other substances. Does a substance change into something completely new when water is added — like sodium metal changes to sodium hydroxide? Does it burn in air?
ü Physical properties: These properties describe the physical characteristics of a substance. The mass, volume, and color of a substance are physical properties, and so is its ability to conduct electricity.
Some physical properties are extensive properties, properties that depend on the amount of matter present. Mass and volume are extensive properties. Intensive properties, however, don’t depend on the amount of matter present. Color is an intensive property. A large chunk of gold, for example, is the same color as a small chunk of gold. The mass and volume of these two chunks are different (extensive properties), but the color is the same. Intensive properties are especially useful to chemists because they can use intensive properties to identify a substance.
How dense are you?
Density is one of the most useful intensive properties of a substance, enabling chemists to more easily identify substances. For example, knowing the differences between the density of quartz and diamond allows a jeweler to check out that engagement ring quickly and easily. Density (d) is the ratio of the mass (m) to volume (v) of a substance. Mathematically, it looks like this:
d = m/v
Usually, mass is described in grams (g) and volume in milliliters (mL), so density is g/mL. Because the volumes of liquids vary somewhat with temperature, chemists also usually specify the temperature at which a density measurement is made. Most reference books report densities at 20°C, because it’s close to room temperature and easy to measure without a lot of heating or cooling. The density of water at 20°C, for example, is lg/mL.
Another term you may sometimes hear is specific gravity (sg), which is the ratio of the density of a substance to the density of water at the same temperature. Specific gravity is just another way for you to get around the problem of volumes of liquids varying with the temperature. Specific gravity is used with urinalysis in hospitals and to describe automobile battery fluid in auto repair shops. Note that specific gravity has no units of measure associated with it, because the units g/mL appear in both the numerator and denominator, canceling each other out (see the “SI/English conversions” section, earlier in this chapter, for info about canceling out units of measure). In most cases, the density and specific gravity are almost the same, so it’s common to simply use the density.
TIP. You may sometimes see density reported as g/cm3 or g/cc. These examples are the same as g/mL. A cube measuring 1 centimeter on each edge (written as 1 cm3) has a volume of 1 milliliter (1 mL). Because 1 mL = 1 cm3, g/mL and g/cm3 are interchangeable. And because a cubic centimeter (cm3) is commonly abbreviated cc, g/cc also means the same thing. (You hear cc a lot in the medical profession. When you receive a 10cc injection, you’re getting 10 milliliters of liquid.)
Measuring density
Calculating density is pretty straightforward. You measure the mass of an object by using a balance or scale, determine the object’s volume, and then divide the mass by the volume.
Determining the volume of liquids is easy, but solids can be tricky. If the object is a regular solid, like a cube, you can measure its three dimensions and calculate the volume by multiplying the length by the width by the height (volume = 1 x w x h). But if the object is an irregular solid, like a rock, determining the volume is more difficult. With irregular solids, you can measure the volume by using something called the Archimedes Principle.
The Archimedes Principle states that the volume of a solid is equal to the volume of water it displaces. The Greek mathematician Archimedes discovered this concept in the third century b.c., and finding an object’s density is greatly simplified by using it. Say that you want to measure the volume of a small rock in order to determine its density. First, put some water into a graduated cylinder with markings for every mL and read the volume. (The example in Figure 2-3 shows 25 mL.) Next, put the rock in, making sure that it’s totally submerged, and read the volume again (29 mL in Figure 2-3). The difference in volume (4 mL) is the volume of the rock.
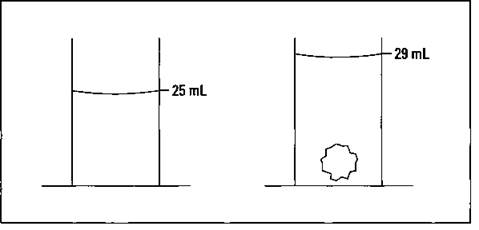
Figure 2-3: Determining the volume of an irregular solid: The Archimedes Principle.
TIP. Anything with a density lower than water will float when put into water, and anything with a density greater than 1 g/mL will sink.
For your pondering pleasure, Table 2-1 lists the density of some common materials.
Table 2-1. Densities of Typical Solids and Liquids in g/mL
Substance |
Density |
Gasoline |
0.68 |
Ice |
0.92 |
Water |
1.00 |
Table Salt |
2.16 |
Iron |
7.86 |
Lead |
11.38 |
Mercury |
13.55 |
Gold |
19.3 |
Energy (Wish I Hart Mere)
Matter is one of two components of the universe. Energy is the other. Energy is the ability to do work. And if you’re like I am, at about 5 p.m. your ability to do work — and your energy level — are pretty low.
Energy can take several forms — such as heat energy, light energy, electrical energy, and mechanical energy. But two general categories of energy are especially important to chemists — kinetic energy and potential energy.
Kinetic energy — moving right along
Kinetic energy is energy of motion. A baseball flying through the air toward a batter has a large amount of kinetic energy. Ask anyone who’s ever been hit with a baseball, and I’m sure that they’ll agree! Chemists sometimes study moving particles, especially gases, because the kinetic energy of these particles helps determine whether a particular reaction may take place. The reason is that collisions between particles and the transfer of energy cause chemical reactions to occur.
The kinetic energy of moving particles can be transferred from one particle to another. Have you ever shot pool? You transfer kinetic energy from your moving pool stick to the cue ball to (hopefully) the ball you’re aiming at.
Kinetic energy can be converted into other types of energy. In a hydroelectric dam, the kinetic energy of the falling water is converted into electrical energy. In fact, a scientific law — The Law of Conservation of Energy — states that in ordinary chemical reactions (or physical processes), energy is neither created nor destroyed but can be converted from one form to another. (This law doesn’t hold in nuclear reactions, though. Chapter 5 tells you why.)
Potential energy — sitting pretty
Suppose you take a ball and throw it up into a tree where it gets stuck. You gave that ball kinetic energy — energy in motion — when you threw it. But where’s that energy now? It’s been converted into the other major category of energy — potential energy.
Potential energy is stored energy. Objects may have potential energy stored in terms of their position. That ball up in the tree has potential energy due to its height. If the ball were to fail, that potential energy would be converted to kinetic energy. (Watch out!)
Potential energy due to position isn’t the only type of potential energy. In fact, chemists really aren’t all that interested in potential energy due to position. Chemists are far more interested in the energy stored (potential energy) in chemical bonds, which are the forces that hold atoms together in compounds.
It takes a lot of energy to run a human body. What if there were no way to store the energy you extract from food? You’d have to eat all the time just to keep your body going. (My wife claims I eat all the time, anyway!) But humans can store energy in terms of chemical bonds. And then later, when we need that energy, our bodies can break those bonds and release it.
The same is true of the fuels we commonly use to heat our homes and run our automobiles. Energy is stored in these fuels — gasoline, for example — and is released when chemical reactions take place.
Measuring Energy
Measuring potential energy can be a difficult task. The potential energy of a ball stuck up in a tree is related to the mass of the ball and its height above the ground. The potential energy contained in chemical bonds is related to the type of bond and the number of bonds that can potentially break.
It’s far easier to measure kinetic energy. You can do that with a relatively simple instrument — a thermometer.
Temperature and temperature scales
When you measure, say, the air temperature in your backyard, you’re really measuring the average kinetic energy (the energy of motion) of the gas particles in your backyard. The faster those particles are moving, the higher the temperature is.
Now all the particles aren’t moving at the same speed. Some are going very fast, and some are going relatively slow, but most are moving at a speed between the two extremes. The temperature reading from your thermometer is related to the average kinetic energy of the particles.
You probably use the Fahrenheit scale to measure temperatures, but most scientists and chemists use either the Celsius (°C) or Kelvin (K) temperature scale. (There’s no degree symbol associated with K.) Figure 24 compares the three temperature scales using the freezing point and boiling point of water as reference points.
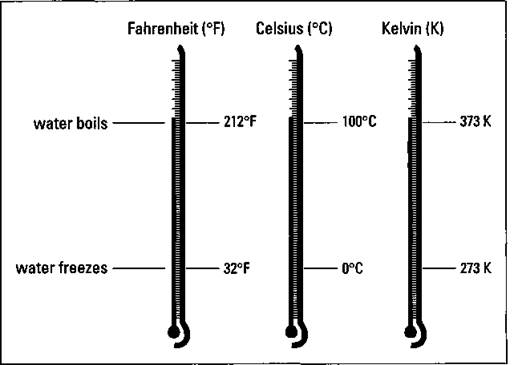
Figure 2-4: Comparison of the Fahrenheit, Celsius, and Kelvin temperature scales.
As you can see from Figure 2-4, water boils at 100°C (373K) and freezes at 0°C (273K). To get the Kelvin temperature, you take the Celsius temperature and add 273. Mathematically, it looks like this:
K = °C + 273
You may want to know how to convert from Fahrenheit to Celsius (because most of us still think in °F). Here are the equations you need:
°C = 5/9 (°F - 32)
Be sure to subtract 32 from your Fahrenheit temperature before multiplying by 5/9.
°F = 9/5 (°C) + 32
Be sure to multiply your Celsius temperature by 9/5 and then add 32.
Go ahead — try these equations out by confirming that the normal body temperature of 98.6°F equals 37°C.
Most of the time in this book, I use the Celsius scale. But when I describe the behavior of gases, I use the Kelvin scale.
Feel the heat
Heat is not the same as temperature. When you measure the temperature of something, you’re measuring the average kinetic energy of the individual particles. Heat; on the other hand, is a measure of the total amount of energy a substance possesses. For example, a glass of water and a swimming pool may be the same temperature, but they contain vastly different amounts of heat. It takes much more energy to raise the temperature of a swimming pool 5°C than it does a glass of water, because there’s so much more water in the swimming pool.
Counting calories
When you hear the word calories, you may think about food and counting calories. Food contains energy (heat). The measure of that energy is the nutritional Calorie (which is commonly capitalized), which is really a kilocalorie (kcal). That candy bar you just ate contained 300 nutritional Calories, which is 300 kcal or 300,000 calories. Thinking of it that way may make it a little easier to resist temptation.
The unit of heat in the SI system is the joule (J). Most of us still use the metric unit of heat, the calorie (cal). Here’s the relationship between the two:
1 calorie = 4.184 joule
The calorie is a fairly small amount of heat — the amount it takes to raise the temperature of 1 gram of water 1°C. I often use the kilocalorie (heal), which is 1,000 calories, as a convenient unit of heat. If you burn a large kitchen match completely, it produces about 1 kilocalorie (1,000 cal) of heat.