Chemistry for Dummies
Part I. Basic Concepts of Chemistry
Chapter 4. The Periodic Table (But No Chairs)
In This Chapter
· Understanding periodicity
· Figuring out how elements are organized in the periodic table
In this chapter, I introduce you to the second most important tool a chemist possesses — the periodic table. (The most important? The beaker and Bunsen burner he or she brews coffee with.)
Chemists are a little lazy, as are most scientists. They like to put things together into groups based on similar properties. This process, called classification, makes it much easier to study a particular system. Scientists have grouped the elements together in the periodic table so they don’t have to learn the properties of individual elements. With the periodic table, they can just learn the properties of the various groups. So in this chapter, I show you how the elements are arranged in the table, and I show you some important groups. I also explain how chemists and other scientists go about using the periodic table.
Repeating Patterns of Periodicity
In nature, as well as in things that mankind invents, you may notice some repeating patterns. The seasons repeat their pattern of fall, winter, spring, and summer. The tides repeat their pattern of rising and falling. Tuesday follows Monday, December follows November, and so on. This pattern of repeating order is called periodicity.
In the mid-1800s, Dmitri Mendeleev, a Russian chemist, noticed a repeating pattern of chemical properties in the elements that were known at the time. Mendeleev arranged the elements in order of increasing atomic mass (see Chapter 3 for a description of atomic mass), to form something that fairly closely resembles our modern periodic table. He was even able to predict the properties of some of the then-unknown elements. Later, the elements were rearranged in order of increasing atomic number, the number of protons in the nucleus of the atom (again, see Chapter 3). Figure 4-1 shows the modern periodic table.
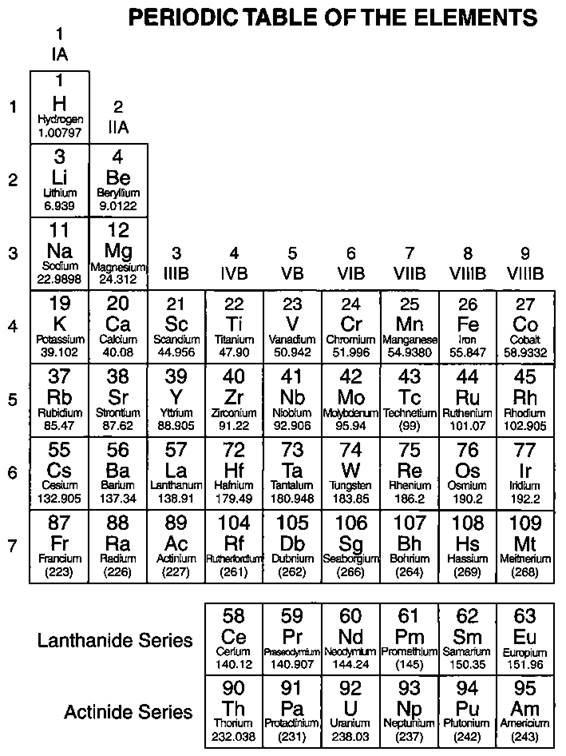
§ Note: Elements 113, 115, and 117 are not known at this time, but are included in the table to show their expected positions.
Figure 4-1: The periodic table.
Chemists can’t imagine doing much of anything without having access to the periodic table. Instead of learning the properties of 109+ elements (more are created almost every year), chemists — and chemistry students — can simply learn the properties of families of elements, thus saving a lot of time and effort. They can find the relationships among elements and figure out the formulas of many different compounds by referring to the periodic table. The table readily provides atomic numbers, mass numbers, and information about the number of valence electrons.
I remember reading a science fiction story many years ago about an alien life based on the element silicon. Silicon was the logical choice for this story because it’s in the same family as carbon, the element that’s the basis for life on earth. So the periodic table is an absolute necessity for chemists, chemistry students, and science fiction novelists. Don’t leave home without it!
Understanding How Elements Are Arranged in the Periodic Table
Look at the periodic table in Figure 4-1. The elements are arranged in order of increasing atomic number. The atomic number (number of protons) is located right above the element symbol. Under the element symbol is the atomic mass, or atomic weight (sum of the protons and neutrons). Atomic mass is a weighted average of all naturally occurring isotopes. (And if that’s Greek to you, just flip to Chapter 3 for tons of fun with atomic mass and isotopes.) Notice also that two rows of elements — Ce-Lu (commonly called the Lanthanides) and Th-Lr (the Actinides) — have been pulled out of the main body of the periodic table. If they were included in the main body of the periodic table, the table would be much larger.
The periodic table is composed of horizontal rows called periods. The periods are numbered 1 through 7 on the left-hand side of the table. The vertical columns are called groups, or families. Members of these families have similar properties (see the section “Families and periods,” later in this chapter). The families may be labeled at the top of the columns in one of two ways. The older method uses Roman numerals and letters. Many chemists (especially old ones like me) prefer and still use this method. The newer method simply uses the numbers 1 through 18.1 use the older method in describing the features of the table.
Using the periodic table, you can classify the elements in many ways. Two quite useful ways are
ü Metals, nonmetals, and metalloids
ü Families and periods
Metals, nonmetals, and metalloids
If you look carefully at Figure 4-1, you can see a stair-stepped line starting at Boron (B), atomic number 5, and going all the way down to Polonium (Po), atomic number 84. Except for Germanium (Ge) and Antimony (Sb), all the elements to the left of that line can be classified as metals. Figure 4-2 shows the metals.
These metals have properties that you normally associate with the metals you encounter in everyday life. They are solid (with the exception of mercury, Hg, a liquid), shiny, good conductors of electricity and heat, ductile (they can be drawn into thin wires), and malleable (they can be easily hammered into very thin sheets). And all these metals tend to lose electrons easily (see Chapter 6). As you can see, the vast majority of the elements on the periodic table are classified as metals.
Except for the elements that border the stair-stepped line (more on those in a second), the elements to the right of the line are classified as nonmetals (along with hydrogen). These elements are shown in Figure 4-3.
Nonmetals have properties opposite those of the metals. The nonmetals are brittle, not malleable or ductile, poor conductors of both heat and electricity, and tend to gain electrons in chemical reactions. Some nonmetals are liquids.
The elements that border the stair-stepped line are classified as metalloids, and they’re shown in Figure 4-4.
The metalloids, or semimetals, have properties that are somewhat of a cross between metals and nonmetals. They tend to be economically important because of their unique conductivity properties (they only partially conduct electricity), which make them valuable in the semiconductor and computer chip industry. (Did you think the term silicon valley referred to a valley covered in sand? Nope. Silicon, one of the metalloids, is used in making computer chips.)
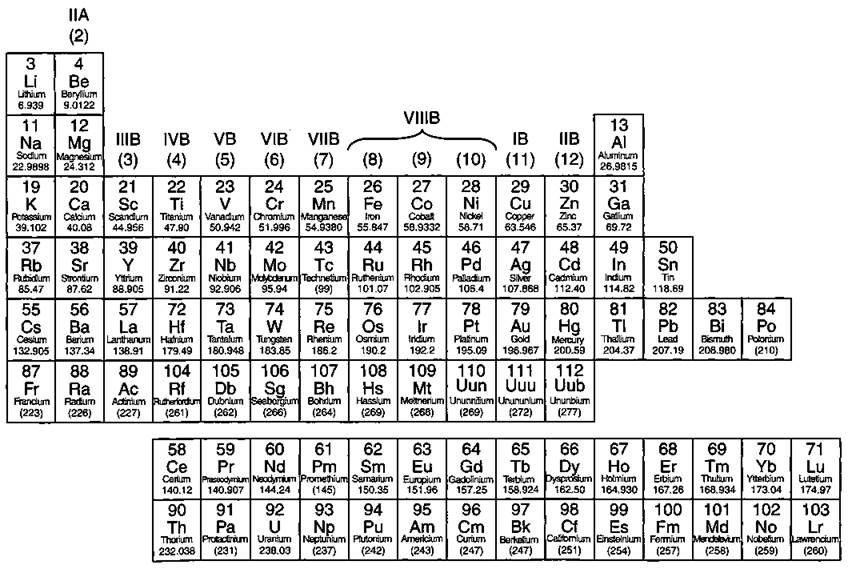
Figure 4-2: The metals.
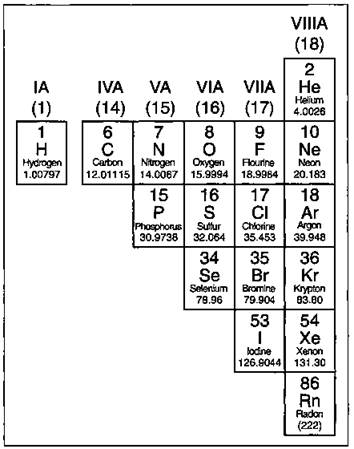
Figure 4-3: The nonmetals.
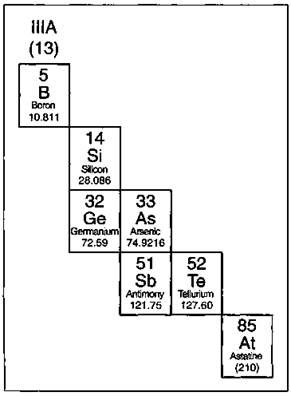
Figure 4-4: The metalloids.
Families and periods
If you refer to the periodic table shown in Figure 4-1, you see seven horizontal rows of elements called periods. In each period, the atomic numbers increase from left to right.
Even though they’re in the same period, these elements have chemical properties that are not all that similar. Consider the first two members of period 3: sodium (Na) and magnesium (Mg). In reactions, they both tend to lose electrons (after all, they are metals), but sodium loses one electron, while magnesium loses two. Chlorine (Cl), down near the end of the period, tends to gain an electron (it’s a nonmetal). So what you need to remember is that members of a period don’t have very similar properties.
The members of a family do have similar properties. Consider the IA family, starting with Lithium (Li) — don’t worry about hydrogen, because it’s unique, and it doesn’t really fit anywhere — and going through Francium (Fr). All these elements tend to lose only one electron in reactions. And all the members of the VIIA family tend to gain one electron.
So why do the elements in the same family have similar properties? And why do some families have the particular properties of electron loss or gain? To find out, you can examine four specific families on the periodic table and look at the electron configurations for a few elements in each family.
My family name is special
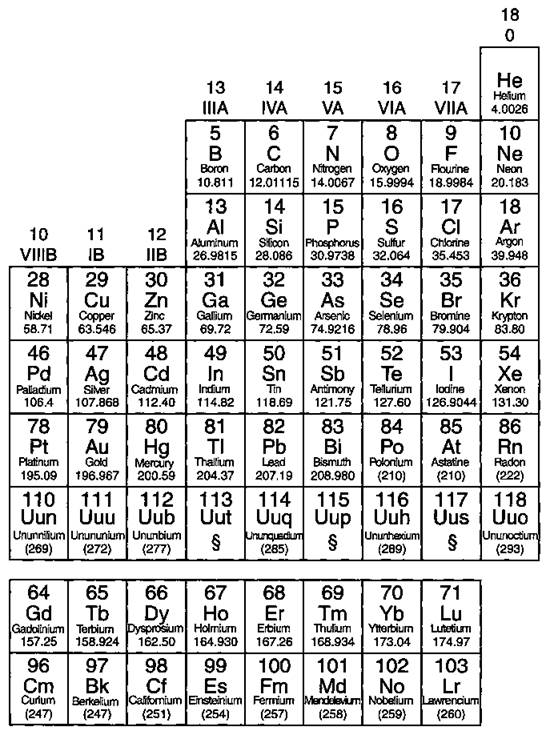
Take a look at Figure 4-5, which lists some important families that are given special names:
ü The IA family is made up of the alkali metals. In reactions, these elements all tend to lose a single electron. This family contains some important elements, such as sodium (Na) and potassium (K). Both of these elements play an important role in the chemistry of the body and are commonly found in salts.
ü The IIA family is made up of the alkaline earth metals. All these elements tend to lose two electrons. Calcium (Ca) is an important member of the IIA family (you need calcium for healthy teeth and bones).
ü The VIIA family is made up of the halogens. They all tend to gain a single electron in reactions. Important members in the family include chlorine (Cl), used in making table salt and bleach, and iodine (T). Ever use tincture of iodine as a disinfectant?
ü The VIIIA family is made up of the noble gases. These elements are very unreactive. For a long time, the noble gases were called the inert gases, because people thought that these elements wouldn’t react at all. Later, a scientist named Neil Bartlett showed that at least some of the inert gases could be reacted, but they required very special conditions. After Bartlett’s discovery, the gases were then referred to as noble gases.
What Valence electrons have to do with families
Chapter 3 explains that an electron configuration shows the number of electrons in each orbital in a particular atom. The electron configuration forms the basis of the concept of bonding and molecular geometry and other important stuff that I cover in the various chapters of this book.
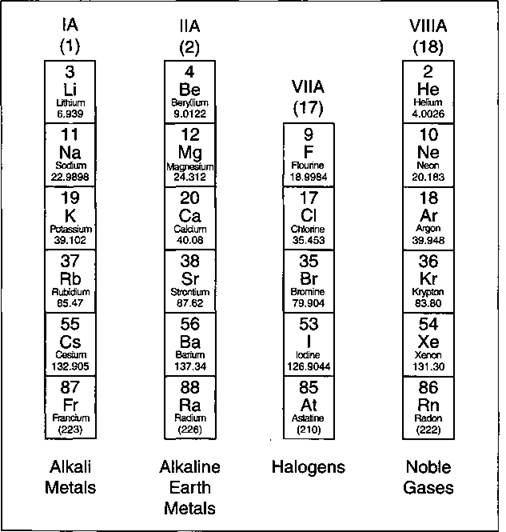
Figure 4-5: Some important chemical families.
Tables 4-1 through 4-4 show the electron configurations for the first three members of the families IA, IIA, VIIA, and VIIIA.
Table 4-1 Electron Configurations for Members of IA (alkali metals)
|
Element |
Electron Configuration |
|
Li |
1s22s1 |
|
Na |
1s22s22p63s1 |
|
K |
1s22s22p63s23p64s1 |
Table 4-2. Electron Configurations for Members of IIA (alkaline earth metals)______
|
Element |
Electron Configuration |
|
Be |
1s22s2 |
|
Mg |
1s22s22p63s2 |
|
Ca |
1s22s22p63s23p64s2 |
Table 4-3. Electron Configurations for Members of VIIA (halogens)
|
Element |
Electron Configuration |
|
F |
1s22s22p5 |
|
Cl |
1s22s22p63s23p5 |
|
Br |
1s22s22p63s23p64s23d104p5 |
Table 4-4. Electron Configurations for Members of VIIIA (noble gases)
|
Element |
Electron Configuration |
|
Ne |
1s22s22p6 |
|
Ar |
1s22s22p63s23p6 |
|
Kr |
1s22s22p63s23p64s23d104p6 |
These electron configurations show that some similarities among each group of elements are in terms of their valence electrons. Valence electrons are the s and p electrons in the outermost energy level of an atom (see Chapter 3).
Look at the electron configurations for the alkali metals (Table 4-1). In lithium, energy level 1 is filled, and a single electron is in the 2s orbital. In sodium, energy levels 1 and 2 are filled, and a single electron is in energy level 3. All these elements have one valence electron in an s orbital. The alkaline earth elements (Table 4-2) each have two valence electrons. The halogens (Table 4-3) each have seven valence electrons (in s and p orbitals — d orbitals don’t count), and the noble gases (Table 4-4) each have eight valence electrons, which fill their valence orbitals.
So how do you remember all this stuff?
REMEMBER. Here’s something to keep in mind about the number of valence electrons and the Roman numeral column number: The IA family has 1 valence electron; the IIA family has 2 valence electrons; the VIIA family has 7 valence electrons; and the VIIIA family has 8 valence electrons. So for the families labeled with a Roman numeral and an A, the Roman numeral gives the number of valence electrons. Pretty cool, eh?
The Roman numeral makes it very easy to determine that oxygen (O) has six valence electrons (it’s in the VIA family), that silicon (Si) has four, and so on. You don’t even have to write the electronic configuration or the energy diagram to determine the number of valence electrons.
Noble and gassy
The fact that the noble gases have eight valence electrons, filling their valence, or outermost energy level, explains why the noble gases are extremely hard to react They are stable, or “satisfied,” with a filled (complete) valence energy level. They don't easily lose, gain, or share electrons.
A lot of stability in nature seems to be associated with this condition. Chemists observe that the other elements in the A families on the periodic table tend to lose, gain, or share valence electrons in order to achieve the goal of having a filled valence shell of eight electrons: This is sometimes called the octet rule. For example, look at the electron configuration for sodium (Na): 1s22s22p63s1. It has one valence electron— the 3s1. If it lost that electron, its valence shell would be energy level 2, which is filled. Without the 3s1, it would become isoelectronic (have the same electronic configuration) as Neon (Ne) and achieve stability. As I show you in Chapters 6 and 7, this is the driving force in chemical bonding: achieving stability by having a filled valence shell.
But what about elements that are labeled with a Roman numeral and a B? These elements, found in the middle of the periodic table, are commonly called the transition metals; their electrons are progressively filling the d orbitals. Scandium (Sc) is the first member of the transition metals, and it has an electronic configuration of 1s22s22p63s23p64s23d1. Titanium (Ti), the next transition metal, has a configuration 1s22s22p63s23p64s23d2. Notice that the number of electrons in the s and p orbitals is not changing. The progressively added electrons fill the d orbitals. Lanthanides and Actinides, the two groups of elements that are pulled out of the main body of the periodic table and shown below it, are classified as inner transition metals. In these elements, the electrons are progressively filling the f orbitals in much the same way that the electrons of the transition metals fill the d orbitals.