Chemistry Essentials for Dummies
Chapter 1. Matter and Energy: Exploring the Stuff of Chemistry
Pure Substances and Mixtures
One of the basic processes in science is classification. In this section, I explain how all matter can be classified as either a pure substance or a mixture (see Figure 2-2).
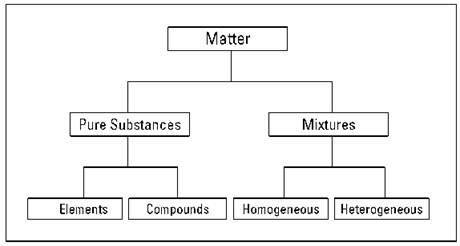
Figure 2-2: Classifying of matter.
Pure substances
REMEMBER. A pure substance, like salt or sugar, has a definite and constant composition or makeup. A pure substance can be either an element or a compound, but the composition of a pure substance doesn’t vary.
Elements
An element is composed of a single kind of atom. An atom is the smallest particle of an element that still has all the properties of the element. For instance, if you slice and slice a chunk of the element gold until only one tiny particle is left that can’t be chopped anymore without losing the properties that make gold gold, then you have an atom. (I discuss properties later in the section “Nice Properties You’ve Got There.”)
The atoms in an element all have the same number of protons. Protons are subatomic particles — particles of an atom. (Chapter 2 covers the three major subatomic particles in great, gory detail.) The important thing to remember right now is that elements are the building blocks of matter. They’re represented in the periodic table, which you explore in Chapter 3.
Compounds
A compound is composed of two or more elements in a specific ratio. For example, water (H2O) is a compound made up of two elements, hydrogen (H) and oxygen (O). These elements are combined in a very specific way — in a ratio of two hydrogen atoms to one oxygen atom (hence, H2O). A lot of compounds contain hydrogen and oxygen, but only one has that special 2-to-1 ratio called water.
REMEMBER. A compound has physical and chemical properties different from the elements that make it up. For instance, even though water is made up of hydrogen and oxygen, water’s properties are a unique combination of the two elements.
Chemists can’t easily separate the components of a compound: They have to resort to some type of chemical reaction.
Throwing mixtures into the mix
REMEMBER. Mixtures are physical combinations of pure substances that have no definite or constant composition; the composition of a mixture varies according to whoever prepares the mixture. Each component of the mixture retains its own set of physical and chemical characteristics.
Chemists can easily separate the different parts of a mixture by physical means, such as filtration. For example, suppose you have a mixture of salt and sand, and you want to purify the sand by removing the salt. You can do this by adding water, dissolving the salt, and then filtering the mixture. You then end up with pure sand.
Mixtures can be either homogeneous or heterogeneous:
✓ Homogeneous mixtures: Sometimes called solutions, homogeneous mixtures are relatively uniform in composition. Every portion of the mixture is like every other portion. If you dissolve sugar in water and mix it really well, your mixture is basically the same no matter where you sample it. I cover solutions in Chapter 10.
✓ Heterogeneous mixtures: The composition of heterogeneous mixtures varies from position to position within the sample. For instance, if you put some sugar in a jar, add some sand, and then give the jar a couple of shakes, your mixture doesn’t have the same composition throughout the jar. Because the sand is heavier, there’s probably more sand at the bottom of the jar and more sugar at the top.