Chemistry Essentials for Dummies
Chapter 8. Electrochemistry: Using Electrons
Exploring Electrochemical Cells
Redox reactions sometimes involve direct electron transfer, in which one substance immediately picks up the electrons another has lost. For instance, if you put a piece of zinc metal into a copper(II) sulfate solution, zinc gives up two electrons (becomes oxidized) to the Cu2+ ion that accepts the electrons (reducing it to copper metal). The copper metal begins spontaneously plating out on the surface of the zinc. The equation for the reaction is
![]()
But if you separate those two half-reactions so that when the zinc is oxidized, the electrons it releases are forced to travel through a wire to get to the Cu2+, you get something useful: a galvanic or voltaic cell, a redox reaction that produces electricity. In this section, I show you how that Zn/Cu2+ reaction may be separated out so that you have an indirect electron transfer and can produce some useable electricity. I also show you how electrolytic cells do the reverse, using electricity to cause a redox reaction. Finally, you see how rechargeable batteries both generate electricity and cause chemical reactions.
Galvanic cells: Getting electricity from chemical reactions
Galvanic cells use redox reactions to produce electricity. These cells are commonly called batteries, but sometimes this name is somewhat incorrect, because a battery is composed of two or more cells connected together. You put a battery in your car, but you put a cell into your flashlight.
A Daniell cell is a type of galvanic cell that uses the Zn/Cu2+ reaction to produce electricity. In the Daniell cell, a piece of zinc metal is placed in a solution of zinc sulfate in one container, and a piece of copper metal is placed in a solution of copper(II) sulfate in another container. These strips of metal are called the cell’s electrodes. They act as a terminal, or a holding place, for electrons.
A wire connects the electrodes, but nothing happens until you put a salt bridge between the two containers. The salt bridge, normally a U-shaped hollow tube filled with a concentrated salt solution, provides a way for ions to move from one container to the other to keep the solutions electrically neutral. It’s like running only one wire up to a ceiling light; the light won’t work unless you put in a second wire to complete the circuit.
With the salt bridge in place, electrons can start to flow through the same basic redox reaction as the one I show you at the beginning of this section. Zinc is being oxidized, releasing electrons that flow through the wire to the copper electrode, where they’re available for the Cu2+ ions to use in forming copper metal. Copper ions from the copper(II) sulfate solution are being plated out on the copper electrode, while the zinc electrode is being consumed. The cations in the salt bridge migrate to the container with the copper electrode to replace the copper ions being consumed, while the anions in the salt bridge migrate toward the zinc side, where they keep the solution containing the newly formed Zn2+ cations electrically neutral.
The zinc electrode is called the anode, the electrode at which oxidation takes place, and it’s labeled with a - sign. The copper electrode is called the cathode, the electrode at which reduction takes place, and it’s labeled with a + sign.
This Daniell cell produces a little over 1 volt. You can get just a little more voltage if you make the solutions that the electrodes are in very concentrated. But what can you do if you want, for example, 2 volts? You have a couple of choices: You can hook two of these cells up together and produce 2 volts, or you can choose two different metals that are farther apart than zinc and copper on the activity series chart (see Chapter 7). The farther apart the metals are on the activity series, the more voltage the cell produces.
Electrolytic cells: Getting chemical reactions from electricity
An electrolytic cell uses electricity to produce a desired redox reaction. For instance, water can be decomposed by the use of electricity in an electrolytic cell. The overall cell reaction is
![]()
In a similar fashion, you can produce sodium metal and chlorine gas by the electrolysis of molten sodium chloride.
Producing chemical changes by passing an electric current through an electrolytic cell is called electrolysis. This reaction may be the recharging of a battery (as you see the next section) or one of many other applications. For instance, ever wonder how the aluminum in that aluminum can is mined? Aluminum ore is primarily aluminum oxide (Al2O3). People produce aluminum metal by reducing the aluminum oxide in a high-temperature electrolytic cell using approximately 250,000 amps. That’s a lot of electricity. Taking old aluminum cans, melting them down, and reforming them into new cans is far cheaper than extracting the metal from the ore. That’s why the aluminum industry is strongly behind the recycling of aluminum. It’s just good business.
Electrolytic cells are also used in a process called electroplating. In electroplating, a more-expensive metal is plated (deposited in a thin layer) onto the surface of a cheaper metal by electrolysis. Back before plastic auto bumpers became popular, chromium metal was electroplated onto steel bumpers. Those five-dollar gold chains you can buy are really made of some cheap metal with an electroplated surface of gold.
Having it both Ways with rechargeable batteries
Redox reactions can be reversed to regenerate the original reactants, allowing people to make rechargeable batteries. Nickel-cadmium (Ni-Cad) and lithium batteries fall into this category, but the most familiar type of rechargeable battery is probably the automobile battery.
The ordinary automobile battery, or lead storage battery, consists of six cells connected in series. The anode of each cell (where oxidation takes place) is lead, and the cathode (where reduction takes place) is lead dioxide (PbO2). The electrodes are immersed in a sulfuric acid (H2SO4) solution. When you start your car, the following cell reactions take place:
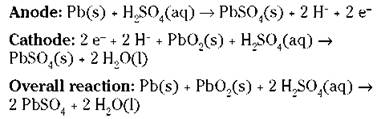
When this happens, both electrodes become coated with solid lead(II) sulfate, and the sulfuric acid is used up.
After you start the automobile, the alternator or generator takes over the job of producing electricity (for spark plugs, lights, and so on) and also recharges the battery. During charging, the automobile battery acts like an electrolytic cell. The alternator reverses both the flow of electrons into the battery and the original redox reactions, and it regenerates the lead and lead dioxide:
![]()
The lead storage battery can be discharged and charged many times. But the shock of running over bumps in the road or into the curb flakes off a little of the lead(II) sulfate and eventually causes the battery to fail.