The Handy Chemistry Answer Book (2014)
INORGANIC CHEMISTRY
ELECTRICITY AND MAGNETISM
What is the difference between paramagnetic and diamagnetic complexes?
In chemistry, atoms or molecules that have at least one unpaired electron (so there is a net spin to the molecule) are known as paramagnetic. If all electrons are paired, chemists refer to the compound as diamagnetic. When a magnetic field is applied a paramagnetic substance will be attracted to the field, while diamagnetic molecules will be repelled from the field.
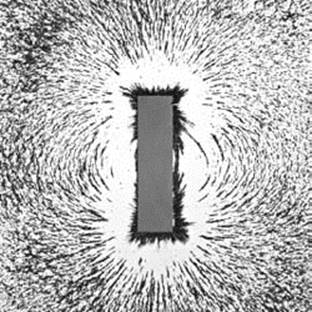
The iron shavings surrounding this magnet provide a good idea of the shape of the magnetic field surrounding this bar magnet, including its north and south poles.
What gives rise to magnetism?
The type of magnetism you’re most familiar with (the kind that keeps magnets on your fridge) is technically known as ferromagnetism. Ferromagnets are permanent magnets—they generate their own magnetic field. Ferromagnets have unpaired electrons (so from the information in the previous question we can say that ferromagnets are paramagnetic, not diamagnetic). But ferromagnets have one additional key trait—the unpaired electron spins are all aligned in the same direction, which generates a permanent magnetic field.

Electric magnets provide both power and a little levitation to maglev trains, resulting in an efficient, smooth, and very fast mode of transportation.
Let’s go through this again from the beginning: Electrons have spin (a quantum mechanical property, but we don’t need to go that far back), and this spin generates a very, very tiny magnetic field. If all the electrons in a substance are paired, it is diamagnetic. If there are unpaired electrons, the substance is paramagnetic. If there are unpaired electrons and those unpaired electrons are aligned so that there is a “net spin” for the (macroscopic) substance, it is a ferromagnetic. Ferromagnets are the magnets you know—they stick to your fridge.
Why are metals attracted to metal?
Or why do magnets stick to your fridge, right? Ferromagnets generate their own magnetic field. This means that other paramagnetic substances will be attracted to ferromagnets, just like they are attracted to magnetic fields. Most metals are paramagnetic, so magnets stick to your metal fridge, but your fridge itself is not magnetic.
So why do opposite ends of magnets attract or repel one another?
Remember that “real world magnets” are ferromagnetic substances—they have a net overall spin. Again this is a quantum mechanical spin, but it’s okay to think of it as spin “up” or “down.” In a ferromagnet all of these spins are pointing in the same direction. If you put the “up” ends of a magnet together, they will repel—the magnetic fields are pushing in opposite directions. If you put an “up” end next to a “down” end, they will attract—the magnetic fields are working in the same direction, just like the electron spins are aligned within the magnet.
Are the North and South Poles of the Earth magnetic poles?
Without getting too technical on the physics here (it gets way more complicated with a whole planet versus a tiny piece of iron on your fridge), yes, the North and South Poles are magnetic poles. But compasses point north, so what we call “The North Pole” is actually the south magnetic pole and vice versa.
What is magnetic levitation?
Magnets apply forces on one another, and these forces can be either attractive or repulsive. If they are repulsive, and if the forces balance against the force of gravity in a carefully designed way, then the magnetic forces can be used to make an object levitate. This is put to practical use in high-speed trains, which can hover above the track while moving quickly on a magnetic cushion.
What makes metals good conductors?
An electrical current is the movement of electrons, so a conductive material allows the free movement of electrons. Metals are good conductors of electricity because of their electronic structure. There are basically two big groups of orbitals in metals—the valence band and the conduction band. The valence band is the group of orbitals that are normally filled in a metal, while the conduction band is empty. These are called bands because they are made up of sets of closely spaced energy levels. Metals, and other good conductors of electricity, can have very small or no band gaps at all. Semiconductors have small band gaps, and electrons can be promoted from the valence band to the conduction band either by heat or light. Insulators, materials that do not conduct electricity, have large band gaps.
What is a band gap?
The band gap for a material is the difference in energy between the valence band and the conduction band. This number tells you how good of a conductor a material is. The smaller the band gap (or the less energy) it takes to promote an electron from the filled to the empty orbitals, the better the material can conduct electrons.
How is a band gap relevant to the design of photovoltaic materials?
The band gap of a material determines what wavelengths of light a photovoltaic (or any other) material can absorb. Photovoltaic materials need to be capable of absorbing the wavelengths of light that the Sun emits, and this requires that they have a band gap that spans the appropriate range of energies.
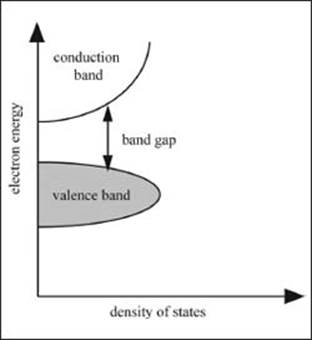
A sample graph showing the band gap of a material between the conduction band and the valence band. The smaller the gap, the better the material is for conducting electricity.