The Handy Chemistry Answer Book (2014)
ATOMS AND MOLECULES
STRUCTURE OF THE ATOM
What is an atom?
Atoms are among the most basic building blocks, making up all matter. The word atom derives from the Greek word atomos, which means “that which cannot be split.” The existence of atoms, or a fundamental, indivisible unit of matter, was proposed long before modern chemistry and physics came about. It turns out that atoms are actually made up of even smaller particles, but the atom is the smallest unit of matter that defines an element. The smaller particles that make up an atom are positively charged protons, charge-neutral neutrons, and negatively charged particles called electrons.
What is an electron?
The electron is a negatively charged subatomic particle, and it is one of three main subatomic particles (the others being the proton and the neutron) that make up atoms. Electrons are responsible for bonding atoms together to make molecules, and they are also the carriers of electric charges in the conducting materials found in the electronic devices you use every day. While protons and neutrons are both found in the center, or nucleus, of an atom, electrons are located apart from the nucleus and are best described as a cloud of electron density. Most reactions in chemistry deal with changes to the arrangement of electrons in some form.
What is a proton?
Protons are subatomic particles that carry a positive charge. They are substantially heavier than electrons (roughly 1,836 times heavier), and carry a positive charge equal in magnitude to that carried by the electron. Protons are found in the nucleus of every atom, and the number of protons present in an atom determines its chemical properties (or, in other words, determines what element it is).
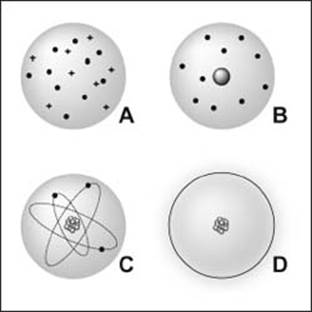
Theoretical models of the atom have evolved over time, including A—the Thomson model (a mix of particles with positive and negative charges), B—the Rutherford model (a positive nucleus surrounded by electrons), C—the Bohr model (stating that electrons follow defined orbits around a nucleus), and D—the quantum mechanical model, based on the idea that you can only determine the probability of an electron’s location.
What is a neutron?
Neutrons are the other principal component of the nucleus of an atom (along with protons). The neutron is neutral in charge and has a mass roughly similar to that of a proton. Atoms of the same element that contain different numbers of neutrons will generally still have the same behavior as one another in terms of chemical reactivity properties. Both protons and neutrons are, in fact, made up of even smaller particles, but chemistry doesn’t usually deal with these even smaller bits.
What were some early models for the atom?
Experiments suggested that atoms were actually made up of smaller particles, which motivated the development of new models involving protons, neutrons, and electrons. One was Thomson’s Plum Pudding Model, which described the atom as a positively charged “pudding” filled with negatively charged electrons. Rutherford later proposed the idea of a positively charged nucleus, but couldn’t explain why electrons didn’t just fall into it. A Danish physicist named Niels Bohr proposed the idea that electrons travel around the nucleus in specific orbits and advanced the atomic theory to a point very close to where it is today.
How did scientists determine that atoms consist of electrons, neutrons, and protons?
Originally atoms were thought to be the smallest unit of matter, but in the late nineteenth century experiments allowed scientists to finally probe inside atoms. Some of these first experiments were carried out by the British physicist J. J. Thomson, who discovered the electron. He noticed that the rays (actually rays of electrons, though he didn’t know it at the time) were deflected by electrically charged plates and concluded that these rays must consist of charged particles that were much smaller than atoms themselves.
Thomson’s first graduate student, Ernest Rutherford, continued to investigate the nature of the atom. In the early twentieth century, Rutherford carried out a now-famous experiment in which radioactive particles were shot through extremely thin gold foil. While some bounced off of the nuclei in different directions, most of the particles actually passed through the foil undeflected. Rutherford interpreted this as an indication that the atoms making up the foil must consist of mostly empty space. Over his career, he developed the picture of the atom as a positively charged center surrounded by electrons, and he also proposed that there must be neutral particles (neutrons) to explain the different isotopes of a given element.
What is the current model for the atom?
The current model for the atom consists of negatively charged electrons orbiting a positively charged nucleus. The nucleus consists of neutrons and protons that are very tightly bound to each other by a strong force. Orbiting electrons behave something like a cloud surrounding the nucleus, and we can’t be sure quite where they are at any given time. The electrons are also very lightweight compared to the nucleus, and they move much, much faster.
What fraction of atoms are empty space?
The fraction of an atom that is occupied by empty space is very large. In fact, over 99.9% of atoms are empty space! The protons, neutrons, and electrons are incredibly small, and the atom occupies such a relatively large effective volume because of the delocalized electron cloud around the nucleus.
What is an atomic mass unit?
One atomic mass unit has a mass of 1.66 × 10−27 kg, which is about the mass of a single proton or neutron. These units are convenient, since when the mass of atoms is expressed in atomic mass units, their masses come out to values that are very close to integers. The values of mass given on the periodic table are expressed in atomic mass units.
What is an isotope?
Isotopes are atoms with the same number of protons and electrons, but with different numbers of neutrons. Since the numbers of protons and electrons effectively determine the reactivity of an atom, isotopes have the same basic chemical properties and are the same element. They have different masses, though, since they have different numbers of neutrons.
The various isotopes of an element are usually present in relatively fixed ratios throughout nature, but in some cases the ratio can depend on the environment or molecule in which they are found. For example, the element carbon most commonly exists with 6 protons, 6 neutrons, and 6 electrons (referred to as Carbon–12, the total number of particles in the nucleus). A small fraction, however, have 6 protons, 7 neutrons, and 6 electrons (Carbon–13). Roughly 99% of carbon atoms have 6 neutrons, but most of the remaining 1% have 7 neutrons.
Below is a table of the breakdown of the isotopic abundance of several common elements: