The Handy Chemistry Answer Book (2014)
NUCLEAR CHEMISTRY

CHEMISTRY INSIDE THE ATOM
How is nuclear chemistry different than other types of chemistry?
As the name implies, nuclear chemistry deals specifically with chemical events involving the nucleus itself, while most other areas of chemistry involve rearrangements of the electrons. Nuclear chemistry is focused on radioactivity and the properties of nuclei, and it finds some of its most important applications in energy production, weapons, and medicine.
What is an isotope?
Isotopes are atoms with the same number of protons and electrons, but with different numbers of neutrons. The most important thing to keep in mind is that the number of protons determines the element we’re dealing with. In most areas of chemistry, this is enough to determine the reactivity of the atom, though in nuclear chemistry the number of neutrons is also quite important in determining the nuclear processes an isotope can undergo. (Please refer back to the “Atoms and Molecules” chapter for additional information on isotopes.)
Do electrons, protons, and neutrons all have the same mass?
Protons, neutrons, and electrons each have different masses. Electrons are, by far, the lightest of the three, with a mass of only about 1/2000th that of a proton or neutron. Protons and neutrons have similar masses, with that of the neutron being just slightly higher than that of the proton. The masses of the three particles in kilograms are:
Electron mass: 9.1094 × 10−31 kg
Proton mass: 1.6726 × 10−27 kg
Neutron mass: 1.6749 × 10−27 kg
Nuclear decay processes can involve the release of electrons, protons, neutrons, or combinations of these basic particles.
Are all isotopes stable?
Not all isotopes of a given element are stable. For example, tin has twenty-two different known isotopes, ten of which are stable and twelve of which are unstable (though there is some debate about just how stable those ten are). Stable is, of course, a relative term. Usually when one says an isotope of an element is stable, it means that it has a decay half-life that is too long to be measured by current methods. There are some elements, such as technetium, radon, and plutonium that do not have any stable known isotopes. In fact, no elements with an atomic number of over 83 (i.e., more than eighty-three protons) have any known isotopes that are considered to be stable!
What is an antiparticle, and what is antimatter?
For most kinds of particles there is postulated to exist a corresponding antiparticle, which is of the same mass but an opposite charge. These antiparticles have only recently been observed in laboratory settings for the first time, and they are very difficult to isolate and study experimentally. This is because particle and antiparticle pairs collide to generate photons of light in a process that annihilates the particle and antiparticle pair. Antiparticles are not well understood and are an active area of research related to nuclear chemistry. Antimatter is just matter made up of antiparticles, in the same way that normal matter is made up of particles. There has been postulated to be an equal amount of matter and antimatter in the universe, though the observations made to date do not suggest this to be the case. This represents an unresolved dilemma that scientists hope to someday better understand. These types of fundamental, unresolved problems are a big part of the reason science is so interesting!
What is a positron?
A positron is the antimatter counterpart of the electron. It has the same mass and spin as an electron, but with a charge opposite in sign and equal in magnitude to that of the electron. If an electron and positron collide, they can annihilate each other and release their energy in the form of a photon.
What are particle accelerators used for?
Particle accelerators are used to generate beams of particles moving at very high speeds, which are typically then collided with matter or other particles to learn about fundamental interactions. Most of the time the particles in question are subatomic particles, though atoms can also be used. Such experiments are used to address fundamental questions in physics surrounding the structure of matter and space. Typical modern particle accelerators are several kilometers long, with some operating in a linear fashion and others in a large ring.
What is a quark?
Quarks are the fundamental particles that make up protons and neutrons, as well as several other types of particles. There are six types of quarks, which are referred to as different “flavors.” These are named up, down, top, bottom, charm, and strange. Protons and neutrons are each made up of three quarks. Two up and one down quark make up a proton. Two down and one up quark make up a neutron.
How do nuclei spontaneously decay?
Nuclei can undergo several types of decay through spontaneous means without colliding or interacting with nuclei of other atoms. The most common types of nuclear decay are called alpha radiation, beta radiation, and gamma radiation. These differ by the type of fragmentation the nucleus undergoes during the decay process.
What is alpha radiation?
Alpha radiation involves the fragmentation of the nucleus into two particles, one consisting of two protons and two neutrons (an alpha particle, or in other words, a helium nucleus), and the other consisting of the remaining protons, neutrons, and electrons initially present in the parent nucleus. Alpha decay decreases the number of protons in the nucleus by two and decreases the atomic mass of the nucleus by four amu.
What is a beta particle?
Beta particles are another type of particle that can be emitted during a nuclear decay process. A beta particle can be either an electron or a positron, which is the antiparticle of an electron. If it is an electron being emitted, one of the neutrons in the nucleus must become a proton to conserve charge in the process. Beta decay increases the number of protons in the nucleus by one and leaves the atomic mass essentially unchanged.
How is nuclear chemistry related to the alchemists’ goal of transmutation?
Alchemists sought a way to turn common metals into gold, which we now know is not possible to do in any simple way. The reason is that transmutation would involve converting one element into another, which can’t be done by simple chemical processes. It would require a nuclear reaction to take place; either a heavy nucleus would have to divide into a gold nucleus and another byproduct, or two lighter nuclei would have to combine to form one of gold. Neither of these things happen readily. If early alchemists had recognized the distinction between more ordinary chemical reactions and the nuclear reaction they were looking for, it would likely have saved a lot of time and effort.
What is gamma radiation?
While alpha and beta radiation is the loss of some particle from an atom, gamma radiation is the release of electromagnetic radiation (called gamma rays). This energy is typically of a high frequency (>1019 Hz), which means it’s high energy (> 100 keV) and can cause significant damage. Gamma radiation can easily penetrate deep into your body, unlike alpha and beta particles, causing damage to your cells and the DNA inside them. Sometimes this damage is useful, though, and some radiation therapies for cancer treatment make use of gamma radiation to kill the malignant cells.
What holds nuclei together?
The nucleus of an atom consists of neutrons, which are uncharged, and protons, which are positively charged. While the uncharged neutrons don’t feel an electrostatic attraction or repulsion to other particles, the positively charged protons should repel each other. In fact, this repulsive force between the protons is quite strong because protons in the same nucleus are very close together. Thus the force that holds them together must be a very strong force. Indeed it is, and it’s even named the strong force. This strong force acts only over distances on the order of 10−15 m—a very very short distance. If the protons were to become separated by a more substantial distance, the strong force would decrease in magnitude faster than the repulsive force, and the protons would be pushed apart. It’s also often said that neutrons act as a sort of “glue” to help bind all of the neutrons and protons together, since there seem to be favored relationships between the number of neutrons and protons present in stable nuclei.
Do all isotopes of an element decay at the same rate?
No, actually each isotope decays at a unique rate. The most radioactive isotopes are those isotopes which decay most quickly. There are some elements (especially the heaviest ones) that don’t have any truly stable isotopes, and these can only be synthesized for fleeting amounts of time in laboratory settings.
What is the half-life of a radioactive species?
The half-life of a radioactive species is the amount of time it takes the quantity of the species to decrease by half. After one half-life, ½ of the initial quantity of material will remain, after two ¼ will remain, after three 1/8 will remain, and so on. Half-lives of radioactive nuclei vary widely, and we’ll list just a few values below to give an idea of the range of timescales covered.
|
Radioactive Nucleus |
Half-Life |
|
Carbon-14 |
5,730 years |
|
Lead-210 |
22.3 years |
|
Mercury-203 |
46.6 days |
|
Lead-214 |
26.8 minutes |
|
Nitrogen-16 |
7.13 seconds |
|
Polonium-213 |
0.000305 seconds |
What defines how long one second lasts?
One second is defined as 9,192,631,770 times the period of the electromagnetic radiation (see the “Physical and Theoretical Chemistry” chapter for more on electromagnetic radiation) corresponding to the difference in hyperfine energy levels in the ground state of a Cesium–133 atom.
What does this mean? To begin, the difference in two closely spaced energy levels of a Cesium–133 atom defines a specific gap in energy. Using the relationship between the energy and frequency of a photon of light, this energy gap can be converted to a frequency of light. Recall that light is electromagnetic radiation. Also recall that the reciprocal of the frequency of light tells us the period of the oscillation of the electromagnetic fields that make up the light. The period tells us how long it takes the electric and magnetic fields to oscillate a single time, and one second is defined as 9,192,631,770 times this (extremely brief) time interval. As those 9,192,631,770 oscillations of the electric field of the light take place, the second hand of each clock on Earth moves 1/60th of a rotation forward.
What is electron capture?
Electron capture is a process that involves an electron combining with a proton to form a neutron. This decreases the atomic number of the element by 1 and leaves the atomic mass unchanged.
Who was Marie Curie?
Marie Curie was a famous French-Polish scientist, and she was the first person ever to be awarded two Nobel prizes, one in chemistry and the other in physics. She was also the first woman to ever win the Nobel Prize, and remains the only woman to have ever won two Nobel prizes in different fields. Curie was responsible for much of the pioneering work in nuclear chemistry during the late nineteenth and early twentieth centuries. Much of her work focused on studying radioactive elements, and she discovered radium and polonium. Tragically, it was Curie’s work that also led to her death. During her career, the dangerous effects of radiation were not yet known, so she worked without the same safety precautions that would be taken today. Her death was the result of a condition known as aplastic anemia, brought on by her prolonged exposure to radiation in the laboratory.
How is radiation exposure quantified?
The scientific unit for radiation exposure is the sievert (Sν), though several other types of units do exist. The maximum radiation exposure that is allowable for occupational exposure in the U.S. is 50 millisieverts (mSν). For comparison, the average natural background level of exposure is roughly 3 mSν.
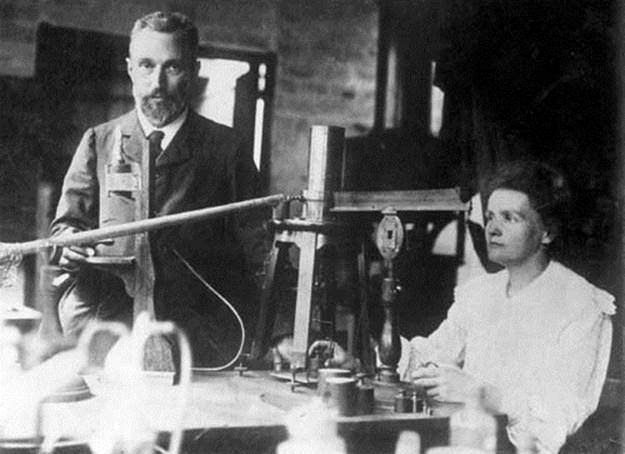
Pierre and Marie Curie working in their laboratory. Marie Curie was the first woman to ever win a Nobel Prize. In fact, she won two of the prestigious prizes. She studied, among other things, radioactive elements and discovered polonium and radium.