The Handy Chemistry Answer Book (2014)
THE MODERN CHEMISTRY LAB
SPECTROSCOPY AND SPECTROMETRY
What does infrared (IR) spectroscopy measure, and what does it tell you about a compound?
Infrared spectroscopy measures the absorption of light by molecules in the infrared region of the spectrum, which provides information on the types of functional groups present in a molecule. For example, looking at the infrared spectrum can provide evidence as to whether certain pairs of atoms are bonded by one, two, or three pairs of electrons. The shapes of the peaks in an infrared spectrum can also be used to gain information about intermolecular interactions between the species we are looking at and the surrounding medium.
How does mass spectrometry (MS) work?
The purpose of mass spectrometry is to ionize a chemical sample, causing it to fragment, and to characterize the mass of the fragment ions that form to gain chemical information about the sample. The first thing that happens is that the sample molecules need to be ionized, and this is done by removing an electron from the sample to yield a positively charged species. Once ionized, a sample of molecules will typically fragment in a characteristic way. The ions that form, whether they are fragments or the original ionized species, are accelerated and then deflected in a magnetic field. Ions of different masses are deflected by different amounts, and this is how the masses of the different ions are distinguished.
Why do mass spectrometers need a vacuum to operate?
As the ions travel, they must not collide with any other atoms or molecules before they reach the detector. If they collide with anything else this will change their direction and kinetic energy, which will make detection either unreliable or impossible.
What is nuclear magnetic resonance (NMR) spectroscopy?
Nuclear magnetic resonance spectroscopy is a type of spectroscopy that involves nuclei in a magnetic field absorbing and re-emitting radiation.
How does NMR help chemists determine the structure of a compound?
The peaks in an NMR spectrum can be related to certain properties of a molecule’s structure. The number of peaks present tells how many types of chemically distinct atoms of a given element are present. A single NMR spectrum typically only contains information about a single element (for organic molecules, hydrogen or carbon NMR spectra are the most common), so it can be useful to record multiple NMR spectra for the same compound. The location of the peaks along the x-axis is known as the chemical shift, and this value can be related to the electron density surrounding each nucleus. In some NMR spectra, the intensities of each peak (their height on the y-axis) can be related to the number of nuclei of a given element that are present. There are more and more details that one learns after spending lots of time interpreting NMR spectra, but these are the basic principles that allow a chemist to relate an NMR spectrum to a chemical structure.
When was NMR invented?
The first time NMR spectra were recorded was in 1945, and two different research groups accomplished this independently (one at Stanford and the other at Harvard). These groups were led by Felix Bloch (Stanford) and Edward Purcell (Harvard), who shared the 1952 Nobel Prize in Physics in recognition of their great discovery. See the list of Nobel Prize winners in Chemistry in the back of this book.
Why do NMR instruments use big magnets?
The purpose of using big magnets is, not surprisingly, to establish a strong, uniform magnetic field in the spectrometer. This creates an energy difference for the nuclear spins that are aligned either with or against the magnetic field. It is this energy difference that is being measured in an NMR spectrum, which can be related to molecular properties like the electron density surrounding a given nucleus.
What compound was the first to be analyzed by proton NMR?
The first recorded example where the shifts of the different nuclei in a molecule were separated by chemical shift was ethanol (CH3CH2OH).
What determines whether a particular type of nucleus can be monitored by NMR spectroscopy?
Similar to electrons, nuclei can have a net “spin,” or spin angular momentum. NMR spectroscopy can be used to study any nucleus that has a nonzero spin angular momentum. Some common nuclei that chemists study by NMR are 1H, 2H (deuterium), 13C, 11B, 15N, 19F, and 31P.
Are only small main group elements detectable by NMR?
The list of nuclei in the previous question are just commonly used ones in NMR experiments. Other elements are observable, but sometimes these require special hardware to obtain useful signal levels. Some of these other nuclei that can be studied by NMR are 17O, 29Si, 33S, 77Se, 89Y, 103Rh, 117Sn, 119Sn, 125Te, 195Pt, 111Cd, 113Cd, 129Xe, 199Hg, 203Tl, 205Tl, and 207Pb.
Is NMR the same as MRI?
MRI, or magnetic resonance imaging, is based on the same principles as NMR spectroscopy. While NMR is typically used to investigate problems related to chemistry and physics, the goal of MRI is to image nuclei in living things. This is accomplished in a way similar to how an NMR spectrum is collected except that the applied magnetic field has a gradient, meaning the field strength is different in different parts of the sample being imaged (usually human or animal tissue). This allows the use of microwave radiation to excite nuclei in individual slices of tissue, one at a time. The gradient in the magnetic field can be varied to collect the MRI of different slices of tissue, which can then be analyzed to get a picture of what is going on inside the body.
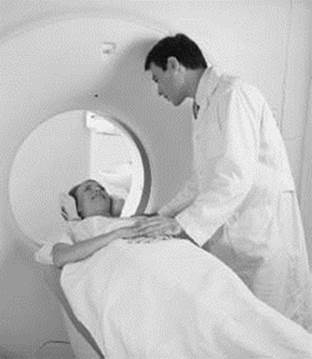
An MRI (magnetic resonance imaging) machine can be used to noninvasively examine the inside of a patient’s body using a magnetic field. MRIs are very useful in diagnosing illnesses such as cancer, the effects of strokes, and torn ligaments.
Who built the first MRI machine?
Dr. Raymond Damadian, who was formally trained as a medical doctor, led construction of the first MRI. The first MRI capable of imaging a human was completed in 1977.
When I get an MRI, what is the machine actually measuring?
An MRI is basically just performing an NMR measurement on the hydrogen atoms in your body. Similar to an NMR instrument, an MRI instrument uses a large magnet and a radio frequency pulse to generate a rotating net magnetization in the hydrogen atoms in your body. The resulting magnetization generates an electric current in a receiver in the MRI instrument that can be processed to generate an image of what’s going on inside your body.