The Handy Chemistry Answer Book (2014)
SUSTAINABLE “GREEN” CHEMISTRY

What is green chemistry?
Green chemistry is the practice of designing chemical products with the goal of minimizing the amount of hazardous waste that is generated in the process. This is frequently a complex issue since one needs to consider not just the synthesis of the chemical product but also the sources from which the reagents are obtained, the manufacture of the product, and the end of the product’s life cycle. In a seminal book on the topic, Paul Anastas and John Warner defined green chemistry as “the utilization of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture, and application of chemical products.”
What are the twelve principles of green chemistry?
The twelve principles of green chemistry, as originally suggested by Paul Anastas and John Warner (and explained by us), are as follows:
Prevention—The best way to minimize the environmental effects of chemical waste is to prevent waste from ever being generated in the first place. It’s easier, and better for the environment, if we can minimize the amount of waste we need to treat, clean up, or store.
Atom Economy—The idea behind this principle is that chemists working on developing synthetic methodologies should generally strive to incorporate as much/many of the reagents as possible into the final product. This helps to prevent the generation of chemical waste and also helps to reduce the quantity of reagents that need to be produced in order to generate the ultimate target.
Less Hazardous Chemical Syntheses—Chemists are encouraged to seek out synthetic routes that avoid or minimize the use of highly toxic chemicals. In addition to trying to minimize the total quantity of waste, we also want to minimize the toxicity of the waste we generate.
Designing Safer Chemicals—In addition to minimizing the toxicity of the waste generated during a chemical synthesis, chemists should strive to select synthetic targets that will also be low in toxicity or have minimal negative impact on the environment.
Safer Solvents and Auxiliaries—Whenever possible, chemists should seek out routes that avoid the use of large quantities of solvents, separation agents, or other chemical auxiliaries. When these cannot be avoided they should be used in minimal quantities, and the choices that are safest for the environment should be made.
Design for Energy Efficiency—The energy costs of synthetic methods should be considered and minimized whenever possible. This may include carrying out reactions and work-up procedures at ambient temperatures and pressures.
Use of Renewable Feedstocks—Reagents and solvents should be obtained from renewable sources whenever possible.
Reduce Derivatives—The number of synthetic steps involving derivatization, such as protection and deprotection steps or use of blocking groups, should be minimized whenever possible. The target of this principle is also to reduce waste and to promote atom economy.
Catalysis—Reagents that behave catalytically, and as selectively as possible, should be chosen over reagents that react stoichiometrically.
Design for Degradation—At the end of their functional life, chemical products should degrade to yield nontoxic products that present minimal threat to the environment.
Real-Time Analysis for Pollution Prevention—Analytical techniques should support in-process monitoring so that the formation of hazardous substances can be prevented.
Inherently Safer Chemistry for Accident Prevention—The use of chemicals should be carried out in a manner that minimizes the likelihood of accidents that may be damaging to the environment or to human health. This includes both the choice of chemicals used as well as the method selected for carrying out a chemical process.
You may notice that the twelve principles are focused closely on the practical synthesis and use of chemical products, and thus they may need to be adapted somewhat depending on the situation (e.g., basic chemical research, large-scale industrial production, nonsynthetic applications of chemistry, etc.).
What are the goals of green chemistry?
If you have read through the twelve principles of green chemistry, you will likely have realized that the focus of green chemistry rests on minimizing the impact of the development, manufacturing, and use of chemical products on the environment. While these twelve principles may not enumerate every possible method for reducing the impact of chemicals on the environment, they provide a foundation for the outcomes that green chemistry seeks to achieve.
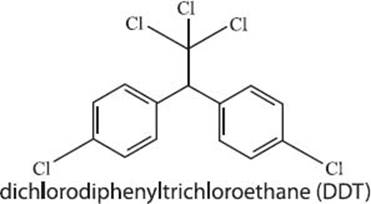
What is DDT?
DDT, or dichlorodiphenyltrichloroethane, is a substance that was widely used as an insecticide until its harmful effects on human health and on wildlife became known; DDT was essentially poisoning the humans and wildlife who came into contact with it. In the context of the advent of green chemistry, DDT carries a special significance in that it helped to awaken the public to the fact that the indiscriminate use of chemicals was causing harm to the environment. News of the harmful effects of DDT was spread by Rachel Carson’s 1962 book, Silent Spring, which explained the numerous negative environmental effects of spraying DDT on crops. Knowledge of the harmful effects of DDT helped to awaken the public to the idea that releasing large amounts of relatively untested chemicals into the environment was potentially causing damage to humans and wildlife. DDT was officially banned in the U.S. in 1972.
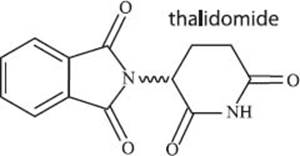
What is thalidomide?
Thalidomide is a drug that was once used to treat the symptoms of morning sickness during pregnancy as well as to help with sleeping problems. A few years after it became widely used, people started to realize that thalidomide was causing birth defects in newborns. These birth defects included phocomelia (abnormal formation of limbs, facial features, nerves, and other parts of the body), problems with sight or hearing resulting from abnormalities in the eyes and ears, gastrointestinal disorders, pasley disorder of the face, underdeveloped lungs, and problems with the digestive tract, heart, and kidneys. The use of thalidomide was then discontinued, though even today there is still some research underway into its possible use in treating cancers. Similar to the situation with DDT, the problems that occurred during the use of thalidomide were particularly influential in motivating the government to tighten regulations on testing drugs and pesticides before their use.
How do you measure how “green” a chemical reaction or process is? What is a life-cycle analysis?
Of course, quantifying the question of “How green is a chemical process?” is not always easy to answer! Even in simply comparing a set of alternative processes, it can still be difficult to determine which is better since each may have advantages and disadvantages for different aspects of human health and the environment.
A life-cycle analysis, or LCA, is a tool used to evaluate and compare the effects of a product on the environment. As the name implies, this includes everything that happens between the time the product is created until it is disposed of. Of course, this is no small task! Typically it involves identifying all relevant materials that go into the production of a product, as well as all of the waste produced during the course of using the product, including things like emissions into the atmosphere, soil, and water, as well as the solid waste produced. Then one needs to evaluate the environmental impact of each of those materials and waste products, hopefully in a manner that allows for the results to readily be compared to those from other products or services. The total of these environmental impacts describes the life-cycle impact of the product.
These inputs and outputs are then converted into their effects or impact on the environment. The sum of these environmental impacts represents the overall environmental effect of the Life Cycle of the product or service. Conducting LCAs for alternative products allows comparison of their overall environmental impacts.
What is bioremediation?
Bioremediation is an approach to removing pollution from the environment that relies on the use of microorganisms to metabolize pollutants into nontoxic products. Sometimes these microorganisms have been genetically engineered for a specific application. For example, a bacterium called deinococcus radiodurans has been genetically engineered to digest ionic mercury compounds and toluene from nuclear waste sites. In such cases, bioremediation can often be accomplished by introducing a microorganism at the site of the pollution, thus avoiding the need for physical cleanup and transportation of the waste to a new location.

Bioremediation is being used to clean up oil-contaminated soil in the Amazon rainforest.
What is bagasse?
After sugarcane stalks are crushed and pressed to remove their juice, the remaining plant matter is known as bagasse. After the water is removed (and bagasse can contain a lot of water), bagasse is mostly composed of cellulose, hemicellulose, and lignin. Typically this material is burned by the sugar mills directly to generate heat for other processes running at the mill or electricity, which can also be used at the mill or sold back to the electric grid. Bagasse also finds its way into paper production. Recently bagasse has been targeted as a potential source of ethanol.
What is phytoremediation?
Some pollutants, such as heavy metals, are not often readily treated by bioremediation techniques. In such cases, phytoremediation may be useful. Phytoremediation relies on the introduction of certain plants that are capable of absorbing a pollutant and concentrating it in the above-ground portion of the plant, which can then be removed. The pollutant-containing plants can then be destroyed in an incinerator to concentrate the pollutants even more, or, in some cases, the pollutants can even be recycled for additional use.
How are plastics sorted for recycling?
The first step in recycling plastics is to sort the plastics by their resin type, or resin identification code. The resin identification code is a number assigned to a plastic product (or container) according to the type of polymers it is made of. While it was once common to directly use this code to identify the types of polymer(s) present, there are now other methods, such as near-infrared spectroscopy or density sorting approaches, that are used to sort mass quantities of plastic samples for recycling. (See “Polymer Chemistry” for more information on resin identification codes.)
What fraction of plastics used in the United States are recycled?
In 2008, about 6.5% of the plastic waste generated was recycled. Roughly another 7.7% was burned to generate energy, while the majority of the remaining waste went into landfills. It is interesting to note that, as plastic production has continued to increase, the fraction of plastics being recycled has decreased. In some cases, this may simply be due to the increasing volume of plastic products or to the fact that it is not easy to build a profitable business around recycling plastics.
What are alternative solvents?
Alternative solvents are relatively environmentally benign solvents that can be substituted for the more hazardous choices, which, while they may be traditionally used, may have established precedence without environmental safety or toxicity concerns in mind.
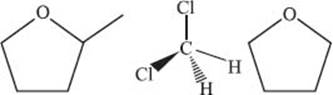
One such example is 2-methyl tetrahydrofuran. As a solvent, it possesses similar characteristics to the widely used dichloromethane and tetrahydrofuran solvents, but with significant environmental advantages in that it is produced from renewable feedstocks, like corn cobs and bagasse, and is easier to separate and clean up.
You can compare the chemical structures of 2-methyl tetrahydrofuran (left) with dichloromethane (center) and tetrahydrofuran (right) below.
How can reactions be run in a solvent-free environment?
There are a few common ways to avoid the use of solvents when running a chemical reaction. The simplest situation is when one of the reagents can serve as the solvent for the reaction. This is commonly referred to as running a reaction “neat” (yes, like scotch). Reagents that are not liquids at ambient temperatures can also be used in the molten state so that they can be used as solvents. Some reactions can also be run on solid-supported catalysts that do not require a solvent. By avoiding the use of solvents, each of these approaches cuts down on costs and on the amount of waste generated.
What are supercritical fluids, and why can these be useful as green solvents?
A supercritical fluid is a substance that has reached sufficiently high temperature and pressure to be beyond a “critical point” on the phase diagram (see “Macroscopic Properties”). This “critical” value of temperature and pressure will be different for each substance, and it corresponds to a set of conditions beyond which the distinction between the liquid and gas phases of matter is no longer clear. That is to say, beyond this value, the density and other properties of the substance can be changed continuously, and readily, with changes in the temperature and pressure of the system.
This makes for useful green solvents because having the ability to tune the density, solubility properties, and diffusivity of the supercritical fluid allows for reaction or extraction conditions to be sensitively manipulated.
Let’s consider one supercritical fluid that has drawn particular interest: carbon dioxide, or CO2. Some of the advantages of CO2 as a supercritical fluid are that it cannot be oxidized, it is aprotic, and it does not tend to participate in reactions involving free radicals. This makes carbon dioxide robust toward undergoing chemical reactions itself, and it also means that it is relatively benign as a contaminant (ignoring its role as a greenhouse gas, for the moment). However, CO2 is a gas at ambient temperature and pressure, and thus, to serve as a good solvent, it must be used at elevated pressures and/or temperatures. At varied temperatures and pressures, supercritical CO2 is also capable of dissolving a wide range of chemical compounds and is miscible with gases in almost any proportion. Supercritical CO2 can also often be recycled as a solvent and thus does not tend to generate large amounts of waste. Indeed, carbon dioxide can also be used as a solvent in its liquid form, but it then loses several of the advantages with regard to the tunability of its properties that we mentioned above.
What is an example of an alternative green reagent?
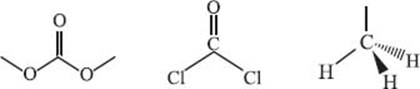
In a similar spirit to alternative solvents, alternative reagents are relatively environmentally benign reagents that are used to replace more toxic ones. One example of an alternative green reagent is dimethyl carbonate, which can be used to effect methylation and carbonylation reactions. Traditionally phosgene or methyl iodide have been used to carry out this same reaction, but the drawback is that these reagents are significantly more toxic and are thus also more costly to dispose of properly. Dimethyl carbonate is a non-toxic compound and it can be readily produced via an oxidative reaction of methanol with oxygen, thus avoiding any environmentally hazardous synthetic procedures.
Chemical structures of dimethyl carbonate (left), phosgene (middle), and a methyl iodide (right):
What is an auxiliary substance?
Chemical auxiliaries are substances like solvents, separation agents, or dispersing agents that are used in the course of a chemical synthesis but are not reagents because they are not incorporated into the chemical product.
Why does the fifth principle of green chemistry seek to avoid the use of auxiliaries?
Ideally one would minimize the use of auxiliary substances used in a chemical synthesis since doing so would generally be expected to reduce the amount of waste produced, thus minimizing the potential for environmental hazards.
Why is heating often used in chemical synthesis?
Heat is often used in chemical synthesis to increase the rate of a reaction. In some cases, heating is also used to effect a phase change. To minimize the environmental impact of a chemical synthesis, it is often optimal to seek reactions that proceed readily at ambient temperatures so that energy input in the form of heating is not necessary.
What is an “E-factor”?
The “E-factor” is a metric of how environmentally friendly, or harmful, a chemical process is. Specifically, the “E-factor” is the ratio of kilograms of waste generated per kilogram of product synthesized. Thus the lower the E-factor, the more environmentally benign a process should be. Of course, this is only a single metric, and other factors, like the toxicity of the waste produced, should also be taken into account. In pharmaceutical companies, the E-factor for the synthesis of drug products is typically in the range of about 25 to 100.
What is an example of how to “green” a chemical process?
At the pharmaceutical giant Pfizer, the synthesis of Viagra® (see “The World Around Us”) originally had an E-factor of 105. However, even before Viagra® was made available to the public, a team of researchers at Pfizer re-examined the entire synthesis step by step. Relatively toxic chlorinated solvents were replaced with less toxic alternatives. The synthesis was also modified to recycle the solvents wherever possible. The use of hydrogen peroxide, which carries some associated health hazards, was removed from the process. Another reagent, oxalyl chloride, is also no longer used; use of this reagent results in the production of carbon monoxide, which is now avoided. In the end, the E-factor for synthesis of Viagra® was reduced to only 8, an over thirteen-fold reduction!
Subsequently, similar changes were made to processes throughout Pfizer. The E-factor for Lyrica®, an anticonvulsant drug, was similarly reduced from an initial value of 86 to now only 9. These sorts of improvements are eliminating millions of tons of chemical waste, while in most cases simultaneously lowering production costs, making safer work conditions, and making products safer for consumers.
How can microwaves be used to promote green chemistry?
Microwaves are electromagnetic radiation in the frequency range of 0.3 to 300 GHz. When microwaves are absorbed by many substances it causes their temperature to increase, thus heating a sample. This is also the same way the microwave in your kitchen heats and cooks food. Microwaves thus offer chemists the opportunity to use heat to promote reactions in cases where conventional heating methods are not possible. This can be useful for promoting reactions under green conditions, such as when we desire to heat the reagents involved in a reaction in the absence of a solvent.
What is the role of photochemical reactions in green chemistry?
Photochemical reactions can often serve as excellent choices for green syntheses. One reason for this is that a photon, unlike chemical catalysts or reagents, leaves behind no waste or excess atoms. Photochemically initiated reactions can often proceed rapidly at ambient temperatures, since photoexcitation can be used to generate highly reactive species. In some cases, photochemical syntheses can also reach the synthetic target in fewer steps than those which rely on thermally initiated reactions.
What are green chemical products?
Green chemical products are products that were designed with the principles of green chemistry in mind so that they will not have harmful effects on human or animal health or on other aspects of the environment. Since the advent of green chemistry, a tremendous number of green products have been developed, ranging from safer household paints to greener cleaning products to new types of plastic products. You may have heard the phrase “benign by design,” which is often used to describe such products.
What are some of the most harmful organic pollutants toward the environment?
The EPA provides a list of twelve particularly persistent organic pollutants to watch out for. The list includes aldrin, chlorodane, dichlorophenyl trichloroethane (DDT), dieldrin, endrin, heptachlor, hexachlorobenzene, mirex, toxaphene, polychlorinated biphenyls, polychlorinated dibenzo-p-dioxins, and polychlorinated dibenzofurans. The EPA has colloquially named this group of compounds as the “dirty dozen.”
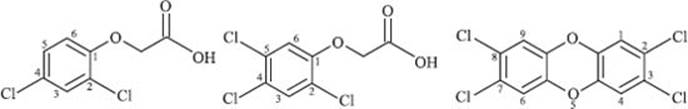
What was Agent Orange?
Agent Orange was an herbicide consisting of a mixture of 2,4-dichlorophenoxyacetic acid and 2,4,5-trichlorophenoxyacetic acid used by the U.S. military during the Vietnam War. The intent was to defoliate rural areas of the country, thus removing strategic ground cover and food sources from the rural areas. It was also discovered that the 2,4,5-trichlorophenoxyacetic acid was contaminated with 2,3,7,8-tetrachlorodibenzodioxin, which is an extremely toxic chemical. Agent Orange was sprayed throughout rural areas of southern Vietnam at high concentrations (an average concentration of thirteen times what was recommended by the USDA for domestic use), resulting in roughly 20% of southern Vietnam’s forests being sprayed. The use of the Agent Orange herbicide resulted in extremely negative health effects for people in these areas, and the effects still persist today despite the Vietnam War having ended in 1975. It is estimated that one million people are currently disabled or suffer major health problems as a result of the use of Agent Orange.
Chemical structures of 2,4-dichlorophenoxyacetic acid (left), 2,4,5-trichlorophenoxyacetic acid (middle), and 2,3,7,8-tetrachlorodibenzodioxin (right):
How has the advent of green chemistry affected the chemical industry and its role in the public sphere?
Over the past several decades, green chemistry has played a crucial role in turning the focus of large chemical companies toward the environmental impact of their products. There are many reasons for this transformation, some of which include government regulations, changing public opinion, and, of course, the desire to preserve and protect the environment. Industrial chemists, government agencies, and the general public alike are now constantly considering the effects of chemicals on the environment, which will hopefully serve to prevent recurrences of chemical-related tragedies like those involving DDT.

The Environmental Protection Agency library is located in Washington, D.C. One of the roles of the EPA is to educate the public about green industry.
What is the role of the EPA in promoting green chemistry?
The United States EPA, or Environmental Protection Agency, has taken great efforts to promote green chemistry. The EPA offers numerous scholarships and awards to promote awareness of green chemistry and the observation of green principles in the chemical industry. It also undertakes efforts to educate the public on green chemistry and, more generally, on the effects of chemical products on human health and on the environment. The EPA also funds research on sustainable technologies and small-business innovation as well as the American Chemical Society Green Chemistry Institute, which promotes partnerships with chemical industry.
What is the role of aqueous hydrogen peroxide as a green reagent?
Hydrogen peroxide, or H2O2, is an ideal choice for a green oxidant because it reacts with high atom efficiency and can react to produce water as the only byproduct. To be a particularly clean oxidant, hydrogen peroxide can be used in aqueous solvents, allowing chemists to avoid the use of any organic solvents. Fortunately there exist catalysts capable of making hydrogen peroxide behave as an efficient oxidant in aqueous conditions, producing products with excellent purity. There are also other reasons that hydrogen peroxide is an ideal green reagent, including the fact that it is relatively inexpensive and is produced in mass quantities. In fact, 2.4 million metric tons of hydrogen peroxide are produced each year. One matter of concern is that high concentrations of hydrogen peroxide can be dangerous, so reactions should be run at concentrations of less than about 60% H2O2.
What is the Warner Babcock Institute for Green Chemistry?
The Warner Babcock Institute for Green Chemistry was founded by John Warner and Jim Babcock to promote the development of environmentally safe and sustainable technologies. The institute offers training in the principles of Green Chemistry for both scientists and nonscientists alike.
Has a Nobel Prize ever been awarded for a discovery in the field of green chemistry?
Yes! Although green chemistry is a relatively young field, one Nobel Prize has already been awarded for work in this area. This prize was awarded in 2005 to three men (Robert Grubbs of the California Institute of Technology, Richard Schrock of the Massachusetts Institute of Technology, and Yves Chauvin of the Institut Francais du Petrole) for their work in the development of olefin metathesis reactions. Olefin metathesis is a type of chemical reaction that involves two carbon-carbon double bonds reacting to form two new carbon-carbon double bonds, effectively exchanging the substituents attached to each carbon. This reaction takes place catalytically under mild reaction conditions, produces little hazardous waste, and has been shown to be effective in a broad range of situations, including the synthesis of new drugs.
Has any legislation been passed regarding the implementation of green chemistry?
Also yes! A couple of examples are the Registration, Evaluation, Authorisation, and Restriction of Chemicals (REACH) program in Europe and the California Green Chemistry Initiative in California in the United States.
The purpose of the REACH program is to require that companies make data available that demonstrates the safety of their products. This includes the potential chemical hazards during the use of a product, and it also describes means of restricting the use of specific chemicals. A similar piece of legislation, the Toxic Substances Control Act, exists in the United States, but this has received criticism for being far less effective.
The California Green Chemistry Initiative was approved in 2008, requiring the California Department of Toxic Substances Control to place priority on specific “chemicals of concern.” This initiative effectively shifted the responsibility for testing chemicals away from individual companies and placed it on the government agency. These laws received criticism for not incentivizing research and education regarding green chemistry in the industry. Due to widespread opposition to the initially proposed regulations, the implementation of this initiative had to be postponed at least once due to the need to rewrite the proposal.
What is the Presidential Green Chemistry Challenge Award?
These awards were created in 1995 in an effort to promote and recognize innovation in green chemistry in the United States. Five awards are given each year to individuals or companies for work in green chemistry in the following categories: Academic, Small Business, Greener Synthetic Pathways, Green Reaction Conditions, and Designing Greener Chemicals.
What are the benefits and challenges of using water as a solvent?
The advantages of using water as a solvent are numerous: water is plentiful, environmentally benign, spans a wide range of temperatures while in the liquid phase, and cuts down on waste. Of course, if there weren’t also some challenges to making it work, we would just be using it for every reaction. A primary challenge of using water is that many compounds are either unstable or insoluble in water. Additionally, many reactions that were developed in organic solvents do not proceed similarly under aqueous conditions for a variety of reasons, so the majority of existing knowledge surrounding organic synthesis (most of which was developed under non-aqueous conditions) often cannot be directly applied to reactivity under aqueous conditions. Water can also be difficult to remove from reactions relative to many organic solvents due to its higher boiling point. Since the advent of green chemistry the amount of research into aqueous synthesis has skyrocketed, and significant progress is being made every day toward the use of water for a growing number of synthetic applications.
What are some examples of biological feedstocks?
It is desirable to use biomass, or plant-based materials, as feedstocks for chemical synthesis and energy production. Through photosynthesis plants are able to efficiently capture and store energy from sunlight, and finding ways to use biomass for green chemistry applications is extremely advantageous in advancing the goals of the field. Sources of biomass can be grouped into several categories, including cellulose, lipids, lignin, terpenes, and proteins. Cellulose is often found in structural parts of plants. Lignin is a polymer often found along with cellulose in woodlike parts of plants. Lipids and lipid oils are often extracted from seeds and soybeans. Terpenes are found in pine trees, rubber trees, and a selection of other plants as well. Proteins are found in relatively small quantities in many types of plants and also in larger quantities in animals. Some efforts are also underway to use genetic transplants to create plants that produce increased amounts of proteins. One of the primary challenges in using biological feedstocks to produce chemicals or energy involves separation and purification of the desired materials.
What was the Bhopal disaster?
The Bhopal disaster (also referred to as the Bhopal gas tragedy) was a gas leak in Bhopal, India, that happened in December of 1984. At the Union Carbide plant in Bhopal, methyl isocyanate gas was accidentally released during a manufacturing process. The gas poisoned thousands of people in the surrounding city, most of whom were asleep when the gas leak occurred. The effects of the release of this gas were felt for years to come, with over half a million injuries reported in the nearly three decades since the incident. Following this accident, criminal and civil suits were filed against the company and several of its highest-ranking employees.

Protesters in Bhopal, India, rage against the injustice of the Union Carbide plant disaster that poisoned thousands with methyl isocyanate gas.
Why is the process of manufacturing ibuprofen an excellent example of green synthesis?
The modern industrial-scale synthesis of ibuprofen has very high atom efficiency, and it has been modified from the original synthesis to be both more environmentally friendly and more cost effective. The original method involved six synthetic steps but used stoichiometric (as opposed to catalytic) quantities of reagents, had lower atom efficiency, and produced undesirable quantities of waste. The modern alternative, on the other hand, requires just three steps, each of which is catalytic in nature. The first step employs a recyclable catalyst (hydrogen fluoride, HF) and produces almost no waste. The second and third steps each achieve 100% atom efficiency (wow!). This process truly represents an ideal benchmark for excellence in green synthesis on the industrial scale.
What is biocatalysis?
Biocatalysis, as the name suggests, involves using enzymes or other natural catalysts to carry out chemical reactions. This tends to work well within the context of green chemistry since biological reactions are often catalyzed in water at mild temperatures and pH values. Moreover, enzymes are themselves environmentally benign and obtained from natural sources. In addition to these benefits, biocatalyzed reactions typically proceed with high selectivity and specificity and require relatively few synthetic steps, thus minimizing the amount of unwanted byproducts produced. Some examples of biocatalyzed syntheses are those of penicillins, cephalosporins (another class of antibiotics), and pregabalin (a drug to relieve pain from damaged nerves). The use of biocatalysis is gaining popularity as the necessary technology becomes more readily available and as more people realize the benefits of this approach.
What are the five environmental spheres?
In the past environmental science focused on the health of four areas, or spheres, of our world. These are the hydrosphere (dealing with water), the atmosphere (dealing with the air), the geosphere (dealing with the Earth), and the biosphere (dealing with living organisms). Environmental scientists have recently been increasingly recognizing a fifth sphere, the anthrosphere, which deals with the ways that humans modify the overall environment by carrying out their daily activities.
What is the greenhouse effect, and how does it affect Earth’s temperature?
The greenhouse effect involves the thermal radiation from the Earth’s surface being absorbed and re-emitted by gases in the atmosphere, termed greenhouse gases. The re-emitted radiation, much of which is in the infrared region of the spectrum, is sent out in all directions, meaning that some of the energy is sent back down toward the lower atmosphere and the Earth’s surface. This results in an overall increase in the surface temperature due to the presence of the greenhouse gases. It should be noted that a certain amount of this greenhouse effect is entirely natural, but the effect can be increased when additional greenhouse gases are introduced into the atmosphere as a result of human activity.
As a side note, the name for this effect arises from the fact that greenhouses allow solar radiation to pass through glass and remain inside. In fact, a greenhouse operates on a different principle; the presence of the glass walls in a greenhouse prevent heat from being lost due to convection currents.
How is rainfall affected by pollutants in the atmosphere?
Air pollution can affect local and global weather patterns, including the amount and frequency of rainfall. At low concentrations particles floating around in the atmosphere may help clouds and thunderstorms to develop, but as their concentration increases these same particles can inhibit the formation of clouds that give rise to rainfall and thunderstorms. This topic is also relevant to issues surrounding climate change, since clouds are understood to generally have a cooling effect on the climate because they reflect incoming sunlight.
What pollutants are introduced into the atmosphere by volcanoes?
Volcanoes are a major source of sulfur dioxide (SO2) gas in the atmosphere. This gas is poisonous and is an irritant to the mucous membranes found in your throat, eyes, and nose. Sulfur dioxide also reacts with oxygen, sunlight, dust, and water to create SO42- droplets and sulfuric acid (H2SO4), which leads to a type of smog referred to as volcanic smog, or “vog.” Vog can cause asthma attacks and damage the upper respiratory tract. The sulfuric acid produced can also cause acid rain.