Industrial Chemistry: For Advanced Students - Mark A. Benvenuto 2015
Tungsten
Tungsten is one of the only handful of elements on the periodic table that has a symbol not derived from a Latin or Greek root word, and a name not derived from either. The symbol, “W” comes from the German word “wolfram,” and the word “tungsten” comes from the Swedish for “heavy stone.” The term “wolfram” in turn comes from the name of the ore wolframite, which apparently comes from old German “Wolf Rahm” meaning “wolf cream.” This rather odd term, in turn, appears to be related to the fact that significant amounts of another metal must be oxidized during the reduction and isolation of tungsten (perhaps turning the other metal into a white oxide).
Since its discovery and isolation in 1783, tungsten has found numerous uses in materials and alloys that require hardness, abrasion resistance, and durability. It remains desirable for these properties today, is tracked by the USGS Minerals Commodity Summaries annually (USGS, 2014), and remains of interest to the world’s militaries and construction industries.
15.1 Mining and sources
There is one tungsten mine in the United States, but the USGS does not report its output, citing company proprietary data. Ignoring this, the world production of tungsten is shown in Figure 15.1, in terms of metric tons. Although now China dominates the world market, simply because of the size of its reserves, the demand for the tungsten mined in Portugal was high immediately before and during the Second World War.
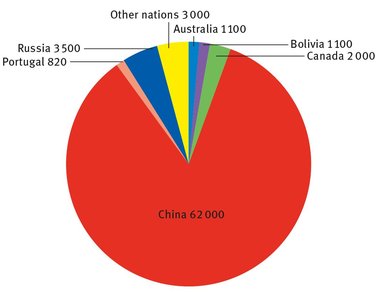
Fig. 15.1: World tungsten production.
This is because China was partially occupied by the armed forces of the Empire of Japan at the time, and Portugal was neutral, and was willing to negotiate with both Allied and Axis nations.
The number of companies and firms that deal with tungsten, from refining, to product development, to sales, is huge. The International Tungsten Industry Association posts a lengthy list of its members on its website (International Tungsten Association, 2013), several of whom also deal with other metals. Additionally, the pie chart does not take into account the amount of tungsten-bearing material that is recycled worldwide. Because of the expense involved in its extraction and purification, this makes up a significant portion of tungsten used each year.
15.2 Extraction chemistry
Tungsten is only found in ores in nature, and not in its reduced form. The minerals wolframite and scheelite are the principle ores from which it is extracted. Table 15.1 gives information on tungsten ores.
The extraction of tungsten involves oxidizing the tungsten in the ores to the +6 state, making it into WO3, and ridding it of impurities. From that point, the material can be reduced. A simplified chemistry is as follows in Figure 15.2.
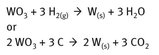
Fig. 15.2: Isolation of tungsten.
Interestingly, scheelite crystals can be made synthetically by what is called the Czochralski process, a process which is more commonly used to make high-purity silicon and semiconductor materials. A melt of the material has a seed crystal introduced, and the seed is slowly drawn from the molten sample. As the cooling material is elevated and drawn, a crystal grows. In the case of scheelite, this is done to produce gem quality stones. In the past, these synthetic gemstones have sometimes been used to simulate diamonds.
Table 15.1: Tungsten ores.
Ore |
General formula |
Comments |
Ferberite |
FeWO4 |
Iron-rich variant of wolframite |
Huebernite |
MnWO4 |
Manganese-rich variant of wolframite |
Scheelite |
CaWO4 |
Major source of W |
Wolframite |
(FeMn)WO4 |
Major source of W |
Table 15.2: Uses for tungsten (International Tungsten Association, 2013).
Item |
Application |
Comments |
Cemented carbide parts |
Wear-resistant and cutting materials |
Usually as tungsten carbide, WC or W2C, with a m.p. =2770°C. |
Heavy alloys |
High-density materials |
Up to 18% W in the alloy. Alloyed with Co, Fe, Ni. |
Electronic components |
Wires, filaments, electrodes |
Used for corrosion resistance. |
Steels |
Superalloys, wear-resistant alloys |
Hastelloy and stellite, for turbine blades. |
|
Chemicals |
WOx ceramic glaze |
|
WS2 lubricant |
Can withstand elevated temperatures. |
|
WO3 catalyst |
15.3 Uses
The profile for the use of tungsten-based materials is broad, but is dominated by the production of cemented carbide parts. A more complete listing is shown in Table 15.2.
The general public often thinks of light bulb filaments as the primary end use product for tungsten metal in a reduced form, but there are uses beyond this for the metal. It is also used in other applications where filaments are required, as well as in what is called tungsten inert gas welding (TIG welding).
15.4 Recycling and reuse
Tungsten is a metal that is recycled from numerous end-use products. The USGS states, “In 2012, the tungsten contained in scrap consumed by processors and end users represented approximately 52% of apparent consumption of tungsten in all forms” (USGS, 2014).
Bibliography
International Tungsten Association. Website. (Accessed 24 December 2013, as: http://www.internationallithium.com. (23—24 Sep 2014, 27th Annual General Meeting)).
USGS Mineral Commodities Summary 2013. Website. (Accessed 20 May, 2014, as: http://minerals.usgs.gov/minerals/pubs/mcs/2013/mcs2013.pdf).