Industrial Chemistry: For Advanced Students - Mark A. Benvenuto 2015
Biofuels and bioplastics
A significant amount of studies have been about biosources of motor fuels (Demibras, 2008; Aresta et al., 2012; Olssen, 2007; Brown and Brown, 2012; Drapcho et al., 2008; Biomass Magazine, 2014; Ethanol Producer Magazine, 2014), and a somewhat smaller number of studies have been carried out concerning the use of biosources to produce chemicals that in turn are used to produce plastics (Pilla, 2011). In both cases, despite a large amount of what can be called proof-of-concept studies, only a few materials have been advanced to the point where they are made into biofuels or bioplastics on an industrial scale. Bio-ethanol is one such fuel. It is almost always made from corn in the United States and sugar cane in Brazil (sugarcane.org, 2014), the two largest national producers of bioethanol. Biodiesel is another, produced mainly from soybeans in Brazil. Plastic from starch is one polymer product that is now an industrial-scale production. Additionally, polylactic acid from biosources is another plastic that can now be made on a large scale from renewable material.
The ability of biosource material to replace petroleum continues to be the subject of heated debate, with production costs, necessary quantities, and the cost of related secondary materials and processes factoring into the debate (Advanced Biofuels Association, 2013; Renewable Fuels Association, 2014; Oil Seed Crops Food and Energy, 2014). Proponents point out the environmental benefits of using renewable materials — as opposed to fossil, petrochemical material sources — for producing fuel and plastics. Detractors point out that fertilizer used for crops such as corn is made from fossil sources. Organizations such as the Renewable Fuels Association indicate that biofuels are more about the economic health of rural communities in the United States than about the environment when they state in their literature, “. . . the ethanol industry continues to have a profoundly positive impact on the fiscal health of rural America” (Renewable Fuels Association, 2014).
26.1 Corn for bioethanol
Undoubtedly, a great deal of effort has been put into converting corn into ethanol for use as a motor fuel. In the United States, fuel blends are in the process of changing from what is called E5 — meaning 5% ethanol in a traditional gasoline blend — to E10. Most fueling stations in the United States have been using E5 gasoline blends for several years (Renewable Fuels Association, 2014; National Corn Growers Association, 2014; American Coalition for Ethanol, 2013). Some stations also offer E85 — which is 85% ethanol — for use in automobiles equipped with engines that are designed to combust this fuel.
The reaction chemistry by which corn is converted into ethanol is shown here in a simplified form in Figure 26.1, utilizing C6H12O6 as a representation for the starch component in corn (which is also used in numerous discussions of this type as a single glucose molecule).
![]()
Fig. 26.1: Ethanol production from corn starch.
Water is absolutely required for this reaction, a fermentation, to occur, but is not shown in the reaction since equi-molar amounts are used and produced. The above reaction can represent any fermentation in which a starch is broken down into ethanol, and indeed, this reaction is a simplified version of that which represents the production of beer or wine. Sources for this fermentation include every plant material that produces sufficient starch, such as corn, sugarcane, fruits, sugar beets, or even wheat and rice. What is sometimes missing from the discussion about ethanol production is the co-production of two moles of carbon dioxide for every starch unit utilized. Critics claim that the co-production of the CO2 contributes to the production of greenhouse gas accumulation, while proponents claim that this CO2 will be reutilized in the production of further crops, and thus is not a net contributor.
26.2 Sugar cane for bioethanol
While bioethanol is routinely made from corn in the United States, in other parts of the world — generally warmer ones — sugarcane is grown easily and can be used as the feedstock for ethanol. Brazil has been producing ethanol from sugarcane for decades, where there is sufficiently warm climate for much of the year, as well as enough arable land on which to cultivate it.
The arguments made about bioethanol production from corn are generally the same as those made when discussing bioethanol from sugarcane. Recently, Shell Oil has formed a collaboration with the Brazilian firm Cosan for this type of fuel production, and states at its website: “This biofuel can reduce CO2 emissions by around 70% compared to standard petrol” (Shell, 2014). Perhaps obviously, detractors will point out that the production of such biofuel comes at a cost of claiming usable land from parts of what had been the Amazon rainforest.
26.3 Soybeans for biodiesel
Diesel is the other major motor fuel produced from some plant material. In the recent past, the term biodiesel has been coined to differentiate between this and the traditional fuel, which is now often called petro—diesel or mineral diesel. As a product, biodiesel is now made on such a large scale that trade organizations exist which are wholly or in part dedicated to its promotion and use (Biomass Magazine, 2014; Biodiesel Magazine, 2014).
Soybeans have become a major plant source for the production of diesel fuel, although biodiesel can also be produced from other plants as well as waste animal fats. The United States Department of Agriculture GAIN Report from 2012 states, “Biodiesel is a trans-esterified vegetable oil also known as fatty acid methyl ester produced from soy oil, rapeseed oil, other vegetable oils, animal fats, and recycled cooking oils” (USDA, 2014). It is these fatty acid methyl esters, often abbreviated FAME, that are the biodiesel, although methanol is not the only alcohol that can be used productively in the trans-esterification. The glycerin that is co-produced is not used as fuel.
Because the plant and animal sources do not have a common starting molecule —like starch for bioethanol production — there will be some variety in the materials that ultimately become biodiesel. In general though, fats or oils are trans-esterified in a reaction that breaks down tri-acyl-glycerides into 3 moles of esters, and a mole of glycerin. The three esters may or may not all be the same, depending on the starting material. A simplified version of this reaction is shown in Figure 26.2.
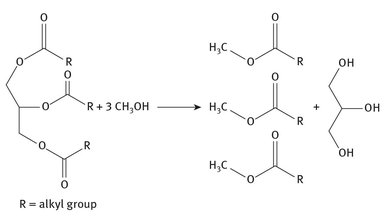
Fig. 26.2: Biodiesel production.
As mentioned, animal sources, usually waste fat from some meat production facility, can be used to produce biodiesel as well. Several poultry processing companies achieved a certain amount of fame in 2007 in the popular press by entering into agreements with diesel producers to use their excess chicken fat for biodiesel. Such waste fat had normally been used in soaps and cosmetics (Washington Post, 2014). These animal-derived sources will produce different fatty esters than plant sources. Examples of two common FAMEs produced in this manner are shown in Figure 26.3.
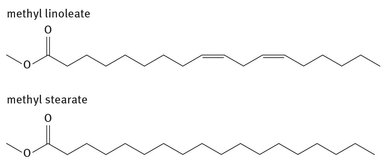
Fig. 26.3: Example fatty acid methyl esters.
26.4 Algae as a fuel source
It may seem odd to consider algae as a viable source for biofuels, but some species of algae are capable of the production of significant amounts of material that can be transformed into biodiesel (National Renewable Energy Laboratory, 2014; Sustainable Development of Algal Biofuels, 2014). These species are capable of producing up to 50% oil based on their weight, and thus there is considerable interest in scaling such production up to an industrial level. Additionally, the possibility exists of using the algal material that does not become biodiesel for the further production of bioethanol. This has been studied rather extensively by the National Renewable Energy Laboratory (NREL) and small-scale production has been proven to be feasible. However, this has not yet progressed to an industrial scale operation (National Renewable Energy Laboratory, 2014).
26.5 Cellulosic bioethanol
A debate continues on the use of corn, sugarcane, soybeans, and other food crops for biofuels, precisely because these crops are staple foods, and the world still has over 1 billion people living in some state of malnourishment. In part because of this, and in part because of the potential market for biofuels from all sources, a great deal of energy has been put into the production of what is called cellulosic ethanol, meaning ethanol produced from biomaterial that is cellulose based as opposed to starch based. This includes corn stover, bagasse, switch grass, and other nonfood plants (Biomass Magazine, 2014; Advanced Biofuels Association, 2013; National Corn Growers Association, 2014).
These sources of bioethanol are collectively referred to as second generation biofuel materials. While several companies are close to starting up cellulosic bioethanol production facilities, all with enzymes that are proprietary, only INEOS Bio had opened a plant at Vero Beach, Florida by the end of 2013 (Renewable Fuels Association, 2014). If the production costs for such operations can be lowered enough, the use of cellulosic ethanol could significantly alter what fuels are offered for automotive use.

Fig. 26.4: Drinking cup made from biodegradable materials.
26.6 Production of bioplastics
More than fuel can be made from bio-based materials. Research has been done for at least the last 30 years on the production of plastics from bio-based sources, with the twin aims being the production of materials from entirely renewable sources and biodegradable plastic materials for consumer use (Corbion Purac, 2014).
The second aim would in theory eliminate the problem of nonbiodegradable plastic materials ending up in landfills. In the recent past, BASF marketed material for waste bags that were manufactured precisely to be biodegradable, but found that sales to consumers ended up being low, which may be a result of the finished product’s appearance or feel (BASF, 2014). In general, it appears that the ecological compatibility and friendliness of such products need to be advertised heavily, as shown in Figure 26.4, two views of a disposable drinking cup made from renewable materials.
26.6.1 Bio-polylactic acid
Polylactic acid (PLA), has recently been brought to market from biofeedstock. Both corn starch and sugarcane starch have been used to produce lactic acid. Several companies that produce it do so while advertising the environmental friendliness of the material. For example, Thyssen—Krupp states at their website: “T-shirts, coffee cups, fast food packaging, bottles and other everyday items: Biobased plastics, also referred to as polylactic acids (PLA), can be used to make a wide variety of products —in environment- and resource-friendly processes” (Thyssen—Krupp, 2014).
The polymerization of the starting material proceeds as is shown in Figure 26.5.
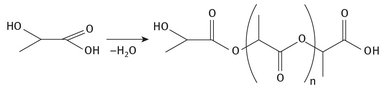
Fig. 26.5: Polylactic acid production.
26.6.2 Bio-poly-starch
Producing polymers from starch actually has a fairly developed history. Starch can be obtained from plant sources, and processed into what is generally called thermoplastic starch. BASF has had success with what it labels Ecoflex®, a starch—polyester blend that biodegrades in only a few weeks (Corbion Purac, 2014).
Such polymers find use in applications as different as packaging “peanuts” and thin films for food storage and preservation. Whether or not their production is profitable and continues to be connected indirectly to the price of crude oil, from which plastic monomers, and ultimately many plastics, are derived.
26.7 Recycling and reuse
Fuels are very seldom recycled, and thus there are no plans for the recycling of biofuels. However, the production of bioplastics is often aimed at producing a plastic material that biodegrades, and thus does not need to be recycled. The fact that a plastic material has been made from biosources as opposed to petroleum sources does not automatically mean that it will be biodegradable, however. In such cases, bioplastics are generally recycled in the same secondary use streams as the petroleum-based plastics. The resin identification codes (RIC codes) 1—7 found on most plastic consumer use products, such as bottles, plates, cups, and plastic cutlery, can be applied to plastics made from biosources as well. This recycling can be a national program, as in some European Union nations, or can be left to smaller governmental bodies, as with the individual states within the United States.
Bibliography
Advanced Biofuels Association. Website. (Accessed 27 December 2013, as: http://advancedbiofuelsassociation.com/ ).
American Coalition for Ethanol. Website. (Accessed 27 December 2013, as: http://www.ethanol.org/).
Aresta, M.; Dibenedetto, A.; Dumeignil, F. (Eds.). “Biorefinery: From Biomass to Chemicals and Fuels,” DeGruyter GmbH, 2012, ISBN: 978-3-11-026023-6.
BASF. Website. (Accessed 5 June 2014, as: http://www.basf.com/group/corporate/us/en/brand/ECOFLEX).
Biodiesel Magazine. (Accessed 1 June 2014, as: http://www.biodieselmagazine.com/).
Bioethanol, DeGruyter, ISSN: 2299-6788.
Biomass Magazine. (Accessed 1 June 2014, as: http://biomassmagazine.com/).
Brown, R. C.; Brown, T. R. “Why are We Producing Biofuels: Shifting to the Ultimate Source of Energy”, Brownia, LLC, 2012, ISBN: 978-0-9840906-3-1.
Corbion Purac. Website. (Accessed 1 June 2014, as: http://www.purac.com/EN/Bioplastics.aspx).
Demibras, A. “Biodiesel: A Realistic Fuel Alternative for Diesel Engines,” Springer-Verlag, 2008, ISBN: 978-1-84628-994-1.
Drapcho, C.; Nghiem, J.; Walker, T. “Biofuels Engineering Process Technology”, McGraw Hill, 2008, ISBN: 978-0-07-148749-8.
Ethanol Producer Magazine. (Accessed 1 June 2014, as: http://www.ethanolproducer.com/).
National Corn Growers Association. Website. (Accessed 1 June 2014, as: http://www.ncga.com/).
National Renewable Energy Laboratory. Website. (Accessed 5 June 2014, as: http://www.nrel.gov/biomass/pdfs/algal_biofuels.pdf). Oil Seed Crops Food and Energy. Website. (Accessed 1 June 2014, as: http://www.oilseedcrops.org).
Olssen, L. (Ed.). “Biofuels,” Springer-Verlag, 2007, ISBN: 978-3-540-73650-9.
Pilla, S. “Handbook of Bioplastics and Biocomposites Engineering Applications,” Scrivener, LLC, 2011, ISBN: 978-0-470-62607-8.
Renewable Fuels Association. Website. (Accessed 4 June 2014, as: http://www.ethanolrfa.org/).
Shell. Website. (Accessed 5 June 2014, as: http://www.shell.com/global/environment-society/environment/climate-change/biofuels-alternative-energies-transport/biofuels/raizen.html).
Sugarcane.org. Website. (Accessed 18 October 2014, as: http://sugarcane.org/sugarcane-products/ethanol).
Sustainable Development of Algal Biofuels in the United States. Website. (Accessed 1 June 2014, as: http://www.nap.edu/catalog.php?record_id=13437).
Thyssen—Krupp. Website. (Accessed 5 June 2014 as: http://www.thyssenkrupp.com/en/produkte/polylactide.html).
USDA. Website. (Accessed 5 June 2014, as: http://gain.fas.usda.gov/Recent%20GAIN%20Publications/Biofuels%20Annual_Sao%20Paulo%20ATO_Brazil_8-21-2012.pdf).
Washington Post. “Not A Tiger, But Maybe A Chicken In Your Tank.” Website. (Accessed 5 June 2014, as: http://www.washingtonpost.com/wp-dyn/content/article/2007/01/02/AR2007010201057.html).