Introductory Chemistry: A Foundation - Zumdahl S.S., DeCoste D.J. 2019
Chemical Foundations: Elements, Atoms, and Ions
Chapter Review
Key Terms
· element symbols (4.2)
· law of constant composition (4.3)
· Dalton’s atomic theory (4.3)
· atom (4.3)
· compound (4.4)
· chemical formula (4.4)
· electron (4.5)
· nuclear atom (4.5)
· nucleus (4.5)
· proton (4.5)
· neutron (4.5)
· isotopes (4.7)
· atomic number (4.7)
· mass number (4.7)
· periodic table (4.8)
· group (4.8)
· alkali metals (4.8)
· alkaline earth metals (4.8)
· halogens (4.8)
· noble gases (4.8)
· transition metals (4.8)
· metals (4.8)
· nonmetals (4.8)
· metalloids (4.8)
· diatomic molecule (4.9)
· ion (4.10)
· cation (4.10)
· anion (4.10)
· ionic compound (4.11)
For Review
· All of the materials in the universe can be chemically broken down into about different elements.
· Nine elements account for about of the earth’s crust, oceans, and atmosphere.
· In the human body, oxygen, carbon, hydrogen, and nitrogen are the most abundant elements.
· Each element has a name and a symbol.
o The symbol usually consists of the first one or two letters of the element’s name.
o Sometimes the symbol is taken from the element’s original Latin or Greek name.
· The law of constant composition states that a given compound always contains the same proportion by mass of the elements of which it is composed.
· Dalton’s atomic theory states:
o All elements are composed of atoms.
o All atoms of a given element are identical.
o Atoms of different elements are different.
o Compounds consist of the atoms of different elements.
o Atoms are not created or destroyed in a chemical reaction.
· A compound is represented by a chemical formula in which the number and type of atoms present are shown by using the element symbols and subscripts.
· Experiments by J. J. Thomson and Ernest Rutherford showed that atoms have internal structure.
o The nucleus, which is at the center of the atom, contains protons (positively charged) and neutrons (uncharged).
o Electrons move around the nucleus.
§ Electrons have a small mass ( of the proton mass).
§ Electrons have a negative charge equal and opposite to that of the proton.
· Isotopes are atoms with the same number of protons but a different number of neutrons.
· A particular isotope is represented by the symbol , in which represents the number of protons (atomic number) and represents the total number of protons and neutrons (mass number) in the nucleus.
· The periodic table shows all of the known elements in order of increasing atomic number; the table is organized to group elements with similar properties in vertical columns.
· Most elements have metallic properties (the metals) and appear on the left side of the periodic table.
· Nonmetals appear on the right side of the periodic table.
· Metalloids are elements that have some metallic and some nonmetallic properties.
· Atoms can form ions (species with a charge) by gaining or losing electrons.
o Metals tend to lose one or more electrons to form positive ions called cations; these are generally named by using the name of the parent atom.
o Nonmetals tend to gain one or more electrons to form negative ions called anions; these are named by using the root of the atom name followed by the suffix -ide.
· The ion that a particular atom will form can be predicted from the atom’s position on the periodic table.
o Elements in Group 1 and 2 form and ions, respectively.
o Group 7 atoms form anions with charges.
o Group 6 atoms form anions with charges.
· Ions combine to form compounds. Compounds are electrically neutral, so the sum of the charges on the anions and cations in the compound must equal zero.
Active Learning Questions
These questions are designed to be considered by groups of students in class. Often these questions work well for introducing a particular topic in class.
· 1.
Knowing the number of protons in the atom of a neutral element enables you to determine which of the following?
a. the number of neutrons in the atom of the neutral element
b. the number of electrons in the atom of the neutral element
c. the name of the element
d. two of the above
e. none of the above
Explain.
· 2.
The average mass of a carbon atom is . Assuming you could pick up one carbon atom, what is the chance that you would randomly get one with a mass of ?
a.
b.
c. about
d.
e. greater than
f. none of the above
Explain.
· 3.
How is an ion formed?
a. by either adding or subtracting protons from the atom
b. by either adding or subtracting neutrons from the atom
c. by either adding or subtracting electrons from the atom
d. all of the above
e. two of the above
Explain.
· 4.
The formula of water, , suggests which of the following?
a. There is twice as much mass of hydrogen as oxygen in each molecule.
b. There are two hydrogen atoms and one oxygen atom per water molecule.
c. There is twice as much mass of oxygen as hydrogen in each molecule.
d. There are two oxygen atoms and one hydrogen atom per water molecule.
e. Two of the above are correct.
Explain.
· 5.
The vitamin niacin (nicotinic acid, ) can be isolated from a variety of natural sources, such as liver, yeast, milk, and whole grain. It also can be synthesized from commercially available materials. Which source of nicotinic acid, from a nutritional view, is best for use in a multivitamin tablet? Why?
· 6.
One of the best indications of a useful theory is that it raises more questions for further experimentation than it originally answered. How does this apply to Dalton’s atomic theory? Give examples.
· 7.
Dalton assumed that all atoms of the same element are identical in all their properties. Explain why this assumption is not valid.
· 8.
How does Dalton’s atomic theory account for the law of constant composition?
· 9.
Which of the following is true about the state of an individual atom?
a. An individual atom should be considered to be a solid.
b. An individual atom should be considered to be a liquid.
c. An individual atom should be considered to be a gas.
d. The state of the atom depends on which element it is.
e. An individual atom cannot be considered to be a solid, liquid, or gas.
For choices you did not pick, explain what you feel is wrong with them, and justify the choice you did pick.
· 10.
These questions concern the work of J. J. Thomson:
a. From Thomson’s work, which particles do you think he would feel are most important in the formation of compounds (chemical changes) and why?
b. Of the remaining two subatomic particles, which do you place second in importance for forming compounds and why?
c. Come up with three models that explain Thomson’s findings and evaluate them. To be complete you should include Thomson’s findings.
· 11.
Heat is applied to an ice cube until only steam is present. Draw a sketch of this process, assuming you can see it at an extremely high level of magnification. What happens to the size of the molecules? What happens to the total mass of the sample?
· 12.
What makes a carbon atom different from a nitrogen atom? How are they alike?
· 13.
Hundreds of years ago, alchemists tried to turn lead into gold. Is this possible? If not, why not? If yes, how would you do it?
· 14.
Chlorine has two prominent isotopes, and . Which is more abundant? How do you know?
· 15.
Differentiate between an atomic element and a molecular element. Provide an example and microscopic drawing of each.
· 16.
Science often develops by using the known theories and expanding, refining, and perhaps changing these theories. As discussed in Section 4.5, Rutherford used Thomson’s ideas when thinking about his model of the atom. What if Rutherford had not known about Thomson’s work? How might Rutherford’s model of the atom have been different?
· 17.
Rutherford was surprised when some of the particles bounced back. He was surprised because he was thinking of Thomson’s model of the atom. What if Rutherford believed atoms were as Dalton envisioned them? What do you suppose Rutherford would have expected, and what would have surprised him?
· 18.
It is good practice to actively read the textbook and to try to verify claims that are made when you can. The following claim is made in your textbook: “ … if the nucleus were the size of a grape, the electrons would be about mile away on average.”
Provide mathematical support for this statement.
· 19.
Why is the term “sodium chloride molecule” incorrect but the term “carbon dioxide molecule” is correct?
· 20.
Both atomic elements and molecular elements exist. Are there such entities as atomic compounds and molecular compounds? If so, provide an example and microscopic drawing. If not, explain why not.
· 21.
Now that you have gone through Chapter 4, go back to Section 4.3 and review Dalton’s Atomic Theory. Which of the premises are no longer accepted? Explain your answer.
· 22.
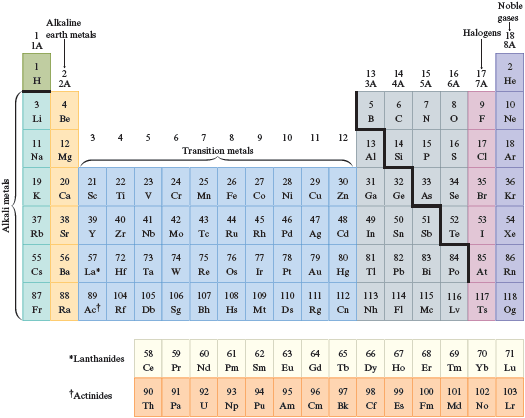
Write the formula for each of the following substances, listing the elements in the order given.
a. 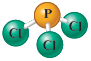
List the phosphorus atom first.
b. a molecule containing two boron atoms and six hydrogen atoms
c. a compound containing one calcium atom for every two chlorine atoms
d. 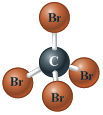
List the carbon atom first.
e. a compound containing two iron atoms for every three oxygen atoms
f. a molecule containing three hydrogen atoms, one phosphorus atom, and four oxygen atoms
· 23.
Use the following figures to identify the element or ion. Write the symbol for each, using the format.
Questions and Problems: 4.1 The Elements
Questions and Problems with answers below also have full solutions in the Student Solutions Guide.
Questions
![]() directs you to the Chemistry in Focus feature in the chapter
directs you to the Chemistry in Focus feature in the chapter
· 1.
What were the four fundamental substances postulated by the Greeks?
· 2.
was the first scientist to recognize the importance of careful measurements.
· 3.
In addition to his important work on the properties of gases, what other valuable contributions did Robert Boyle make to the development of the study of chemistry?
· 4.
What are the three most abundant elements (by mass) in the human body?
· 5.
What are the five most abundant elements (by mass) in the earth’s crust, oceans, and atmosphere?
· 6.
![]() Read the “Chemistry in Focus” segment Trace Elements: Small but Crucial, and answer the following questions.
Read the “Chemistry in Focus” segment Trace Elements: Small but Crucial, and answer the following questions.
a. What is meant by the term trace element?
b. Name two essential trace elements in the body and list their function(s).
Questions and Problems: 4.2 Symbols for the Elements
Questions and Problems with answers below also have full solutions in the Student Solutions Guide.
Questions
Note: Refer to Fig. 4.9 when appropriate.
· 7.
Give the symbols and names for the elements whose chemical symbols consist of only one letter.
· 8.
The symbols for most elements are based on the first few letters of the respective element’s common English name. In some cases, however, the symbol seems to have nothing to do with the element’s common name. Give three examples of elements whose symbols are not directly derived from the element’s common English name.
· 9.
Find the symbol in Column 2 for each name in Column 1.
|
Column 1 |
Column 2 |
||
a. |
helium |
1. |
|
b. |
sodium |
2. |
|
c. |
silver |
3. |
|
d. |
sulfur |
4. |
|
e. |
bromine |
5. |
|
f. |
potassium |
6. |
|
g. |
neon |
7. |
|
h. |
barium |
8. |
|
i. |
cobalt |
9. |
|
j. |
carbon |
10. |
|
11. |
|||
12. |
|||
13. |
|||
14. |
|||
· 10.
Several elements have chemical symbols beginning with the letter . For each of the following chemical symbols, give the name of the corresponding element.
a.
b.
c.
d.
e.
f.
g.
· 11.
Use the periodic table shown in Fig. 4.9 to find the symbol or name for each of the following elements.
Symbol |
Name |
rubidium |
|
radium |
|
· 12.
Use the periodic table inside the front cover of this book to find the symbol or name for each of the following elements.
Symbol |
Name |
zirconium |
|
selenium |
|
cerium |
· 13.
For each of the following chemical symbols, give the name of the corresponding element.
a.
b.
c.
d.
e.
f.
g.
h.
· 14.
Several chemical elements have English names beginning with the letters , , , or . For each letter, list the English names for two elements whose names begin with that letter, and give the symbols for the elements you choose (the symbols do not necessarily need to begin with the same letters).
Questions and Problems: 4.3 Dalton’s Atomic Theory
Questions and Problems with answers below also have full solutions in the Student Solutions Guide.
Questions
· 15.
A given compound always contains the same proportion (by mass) of the elements. This principle became known as .
· 16.
Correct each of the following misstatements from Dalton’s atomic theory.
a. Elements are made of tiny particles called molecules.
b. All atoms of a given element are very similar.
c. The atoms of a given element may be the same as those of another element.
d. A given compound may vary in the relative number and types of atoms depending on the source of the compound.
e. A chemical reaction may involve the gain or loss of atoms as it takes place.
Questions and Problems: 4.4 Formulas of Compounds
Questions and Problems with answers below also have full solutions in the Student Solutions Guide.
Questions
· 17.
What is a compound?
· 18.
A given compound always contains the same relative masses of its constituent elements. How is this related to the relative numbers of each kind of atom present?
· 19.
Based on the following word descriptions, write the formula for each of the indicated substances.
a. a compound whose molecules each contain six carbon atoms and six hydrogen atoms
b. an aluminum compound in which there are three chlorine atoms for each aluminum atom
c. a compound in which there are two sodium atoms for every sulfur atom
d. a compound whose molecules each contain two nitrogen atoms and four oxygen atoms
e. a compound in which there is an equal number of sodium, hydrogen, and carbon atoms, but there are three times as many oxygen atoms as atoms of the other three elements
f. a compound that has equal numbers of potassium and iodide atoms
· 20.
Based on the following word descriptions, write the formula for each of the indicated substances.
a. a compound whose molecules contain twice as many oxygen atoms as carbon atoms
b. a compound whose molecules contain an equal number of carbon and oxygen atoms
c. a compound in which there is an equal number of calcium and carbon atoms, but there are three times as many atoms of oxygen as of the other two elements
d. a compound whose molecules contain twice as many hydrogen atoms as sulfur atoms and four times as many oxygen atoms as sulfur atoms
e. a compound in which there are twice as many chlorine atoms as barium atoms
f. a compound in which there are three sulfur atoms for every two aluminum atoms
Questions and Problems: 4.5 The Structure of the Atom
Questions and Problems with answers below also have full solutions in the Student Solutions Guide.
Questions
· 21.
Scientists J. J. Thomson and William Thomson (Lord Kelvin) made numerous contributions to our understanding of the atom’s structure.
a. Which subatomic particle did J. J. Thomson discover, and what did this lead him to postulate about the nature of the atom?
b. William Thomson postulated what became known as the “plum pudding” model of the atom’s structure. What did this model suggest?
· 22.
True or false? Rutherford’s bombardment experiments with metal foil suggested that the particles were being deflected by coming near a large, positively charged atomic nucleus.
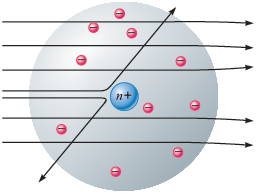
o True
o False
Questions and Problems: 4.6 Introduction to the Modern Concept of Atomic Structure
Questions and Problems with answers below also have full solutions in the Student Solutions Guide.
Questions
· 23.
Where are neutrons found in an atom? Are neutrons positively charged, negatively charged, or electrically uncharged?
· 24.
What are the positively charged particles found in the nuclei of atoms called?
· 25.
Do the proton and the neutron have exactly the same mass? How do the masses of the proton and the neutron compare to the mass of the electron? Which particles make the greatest contribution to the mass of an atom? Which particles make the greatest contribution to the chemical properties of an atom?
· 26.
The proton and the (electron/neutron) have almost equal masses. The proton and the (electron/neutron) have charges that are equal in magnitude but opposite in nature.
· 27.
An average atomic nucleus has a diameter of about m.
· 28.
Which particles in an atom are most responsible for the chemical properties of the atom? Where are these particles located in the atom?
Questions and Problems: 4.7 Isotopes
Questions and Problems with answers below also have full solutions in the Student Solutions Guide.
Questions
![]() directs you to the Chemistry in Focus feature in the chapter
directs you to the Chemistry in Focus feature in the chapter
· 29.
Explain what we mean when we say that a particular element consists of several isotopes.
· 30.
True or false? The mass number of a nucleus represents the number of protons in the nucleus.
o True
o False
· 31.
For an isolated atom, why do we expect the number of electrons present in the atom to be the same as the number of protons in the nucleus of the atom?
· 32.
Why do we not necessarily expect the number of neutrons in the nucleus of an atom to be the same as the number of protons?
· 33.
Dalton’s original atomic theory proposed that all atoms of a given element are identical. Did this turn out to be true after further experimentation was carried out? Explain.
· 34.
Which scientist discovered that the nuclei of most atoms contain neutrons as well as protons?
· 35.
For each of the following elements, use the periodic table shown in Fig. 4.9 to write the element’s atomic number, symbol, or name.
Atomic Number |
Symbol |
Name |
phosphorus |
||
zinc |
· 36.
Of the following isotopes, which are isotopes of the same element?
a.
b.
c.
d.
e.
· 37.
Write the atomic symbol for each of the isotopes described below.
a. the isotope of carbon with neutrons
b. the isotope of carbon with neutrons
c. ,
d. atomic number , mass number
e. ,
f. the isotope of boron with mass number
· 38.
Write the atomic symbol for each of the isotopes described below.
a. ,
b. the isotope of iron with neutrons
c. ,
d. the isotope of nitrogen with neutrons
e. ,
f. atomic number ,
· 39.
How many protons and neutrons are contained in the nucleus of each of the following atoms? Assuming each atom is uncharged, how many electrons are present?
a.
b.
c.
d.
e.
f.
· 40.
![]() Read the “Chemistry in Focus” segment “Whair” Do You Live? How can isotopes be used to identify the general region of a person’s place of residence?
Read the “Chemistry in Focus” segment “Whair” Do You Live? How can isotopes be used to identify the general region of a person’s place of residence?
· 41.
![]() Read the “Chemistry in Focus” segment Isotope Tales. Define the term isotope, and explain how isotopes can be used to answer scientific and historical questions.
Read the “Chemistry in Focus” segment Isotope Tales. Define the term isotope, and explain how isotopes can be used to answer scientific and historical questions.
· 42.
Complete the following table.
Name |
Symbol |
Atomic Number |
Mass Number |
Number of Neutrons |
iron |
||||
cobalt |
||||
chromium |
Questions and Problems: 4.8 Introduction to the Periodic Table
Questions and Problems with answers below also have full solutions in the Student Solutions Guide.
Questions
![]() directs you to the Chemistry in Focus feature in the chapter
directs you to the Chemistry in Focus feature in the chapter
· 43.
True or false? The elements are arranged in the periodic table in order of increasing mass.
· 44.
In which direction on the periodic table, horizontal or vertical, are elements with similar chemical properties aligned? What are families of elements with similar chemical properties called?
· 45.
List the characteristic physical properties that distinguish the metallic elements from the nonmetallic elements.
· 46.
Where are the metallic elements found on the periodic table? Are there more metallic elements or nonmetallic elements?
· 47.
Most, but not all, metallic elements are solids under ordinary laboratory conditions. Which metallic elements are not solids?
· 48.
List five nonmetallic elements that exist as gaseous substances under ordinary conditions. Do any metallic elements ordinarily occur as gases?
· 49.
Under ordinary conditions, only a few pure elements occur as liquids. Give an example of a metallic and a nonmetallic element that ordinarily occur as liquids.
· 50.
The elements that lie close to the “stair-step” line as shown below in blue are called .
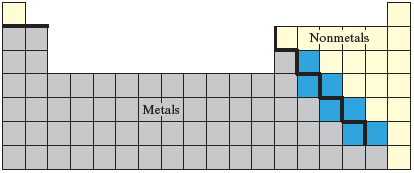
· 51.
Write the number and name (if any) of the group (family) to which each of the following elements belongs.
a. cesium
b.
c.
d. chlorine
e. strontiume
f.
g.
· 52.
Without looking at your textbook or the periodic table, name three elements in each of the following groups (families).
a. halogens
b. alkali metals
c. alkaline earth metals
d. noble/inert gases
· 53.
For each of the following elements, use the periodic table shown in Fig. 4.9 to give the chemical symbol, atomic number, and group number and to specify whether each element is a metal, nonmetal, or metalloid.
a. strontium
b. iodine
c. silicon
d. cesium
e. sulfur
· 54.
![]() The “Chemistry in Focus” segment Putting the Brakes on Arsenic discusses the dangers of arsenic and a possible help against arsenic pollution. Is arsenic a metal, a nonmetal, or a metalloid? What other elements are in the same group on the periodic table as arsenic?
The “Chemistry in Focus” segment Putting the Brakes on Arsenic discusses the dangers of arsenic and a possible help against arsenic pollution. Is arsenic a metal, a nonmetal, or a metalloid? What other elements are in the same group on the periodic table as arsenic?
Questions and Problems: 4.9 Natural States of the Elements
Questions and Problems with answers below also have full solutions in the Student Solutions Guide.
Questions
· 55.
Most substances are composed of rather than elemental substances.
· 56.
Are most of the chemical elements found in nature in the elemental form or combined in compounds? Why?
· 57.
The noble gas present in relatively large concentrations in the atmosphere is .
· 58.
Why are the elements of Group 8 referred to as the noble or inert gas elements?
· 59.
Molecules of nitrogen gas and oxygen gas are said to be , which means they consist of pairs of atoms.
· 60.
Give three examples of gaseous elements that exist as diatomic molecules. Give three examples of gaseous elements that exist as monatomic species.
· 61.
A simple way to generate elemental hydrogen gas is to pass through water.
· 62.
If sodium chloride (table salt) is melted and then subjected to an electric current, elemental gas is produced, along with sodium metal.
· 63.
Most of the elements are solids at room temperature. Give three examples of elements that are liquids at room temperature, and three examples of elements that are gases at room temperature.
· 64.
The two most common elemental forms of carbon are diamond and .
Questions and Problems: 4.10 Ions
Questions and Problems with answers below also have full solutions in the Student Solutions Guide.
Questions
· 65.
An isolated atom has a net charge of .
· 66.
Ions are produced when an atom gains or loses .
· 67.
A simple ion with a charge (for example, ) results when an atom (gains/loses) electrons.
· 68.
An ion that has two more electrons outside the nucleus than there are protons in the nucleus will have a charge of .
· 69.
Positive ions are called , whereas negative ions are called .
· 70.
Simple negative ions formed from single atoms are given names that end in .
· 71.
Based on their location in the periodic table, give the symbols for three elements that would be expected to form positive ions in their reactions.
· 72.
True or false? and contain a different number of protons but the same number of electrons. Justify your answer.
o True
o False
· 73.
How many electrons are present in each of the following ions?
a.
b.
c.
d.
e.
f.
· 74.
State the number of protons, electrons, and neutrons for .
· 75.
For the following processes that show the formation of ions, use the periodic table to indicate the number of electrons and protons present in both the ion and the neutral atom from which the ion is made.
a.
b.
c.
d.
e.
f.
· 76.
For the following ions, indicate whether electrons must be gained or lost from the parent neutral atom, and how many electrons must be gained or lost.
a.
b.
c.
d.
e.
f.
· 77.
For each of the following atomic numbers, use the periodic table to write the formula (including the charge) for the simple ion that the element is most likely to form.
a.
b.
c.
d.
e.
f.
· 78.
On the basis of the element’s location in the periodic table, indicate what simple ion each of the following elements is most likely to form.
a.
b.
c.
d.
e.
f.
Questions and Problems: 4.11 Compounds That Contain Ions
Questions and Problems with answers below also have full solutions in the Student Solutions Guide.
Questions
· 79.
List some properties of a substance that would lead you to believe it consists of ions. How do these properties differ from those of nonionic compounds?
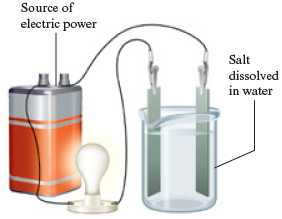
· 80.
Why does a solution of sodium chloride in water conduct an electric current?
· 81.
Why does an ionic compound conduct an electric current when the compound is melted but not when it is in the solid state?
· 82.
Why must the total number of positive charges in an ionic compound equal the total number of negative charges?
· 83.
For each of the following positive ions, use the concept that a chemical compound must have a net charge of zero to predict the formula of the simple compounds that the positive ions would form with the , , and ions.
a.
b.
c.
d.
e.
· 84.
For each of the following negative ions, use the concept that a chemical compound must have a net charge of zero to predict the formula of the simple compounds that the negative ions would form with the , , and ions.
a.
b.
c.
d.
e.
Additional Problems
![]() directs you to the Chemistry in Focus feature in the chapter
directs you to the Chemistry in Focus feature in the chapter
· 85.
For each of the following elements, give the chemical symbol and atomic number.
a. astatine
b. xenon
c. radium
d. strontium
e. lead
f. selenium
g. argon
h. cesium
· 86.
Give the group number (if any) in the periodic table for the elements listed in Problem 85. If the group has a family name, give that name.
· 87.
List the names, symbols, and atomic numbers of the top four elements in Groups 1, 2, 6, and 7.
· 88.
Which of the following statements is(are) true?
a. Dalton was the first to theorize that atoms consist of smaller particles called electrons, protons, and neutrons.
b. Dalton’s atomic theory didn’t account for isotopes.
c. All particles in the nucleus of an atom are charged.
d. The number of neutrons in a neutral atom must equal the number of electrons.
· 89.
Which of the following is(are) true regarding and ?
a. same group number on the periodic table
b. same number of protons
c. same number of neutrons
d. same number of electrons
· 90.
Which subatomic particles contribute most to the atom’s mass? Which subatomic particles determine the atom’s chemical properties?
· 91.
Is it possible for the same two elements to form more than one compound? Is this consistent with Dalton’s atomic theory? Give an example.
· 92.
Carbohydrates, a class of compounds containing the elements carbon, hydrogen, and oxygen, were originally thought to contain one water molecule for each carbon atom present. The carbohydrate glucose contains six carbon atoms. Write a general formula showing the relative numbers of each type of atom present in glucose.
· 93.
When iron rusts in moist air, the product is typically a mixture of two iron—oxygen compounds. In one compound, there is an equal number of iron and oxygen atoms. In the other compound, there are three oxygen atoms for every two iron atoms. Write the formulas for the two iron oxides.
· 94.
How many protons and neutrons are contained in the nucleus of each of the following atoms? For an atom of the element, how many electrons are present?
a.
b.
c.
· 95.
Though the common isotope of aluminum has a mass number of , isotopes of aluminum have been isolated (or prepared in nuclear reactors) with mass numbers of , , , , , and . How many neutrons are present in each of these isotopes? Why are they all considered aluminum atoms, even though they differ greatly in mass? Write the atomic symbol for each isotope.
· 96.
The principal goal of alchemists was to convert cheaper, more common metals into gold. Considering that gold had no particular practical uses (for example, it was too soft to be used for weapons), why do you think early civilizations placed such emphasis on the value of gold?
· 97.
How did Robert Boyle define an element?
· 98.
How many electrons are present in each of the following ions?
a.
b.
c.
d.
e.
f.
· 99.
Give the chemical symbol for each of the following elements.
a. barium
b. potassium
c. cesium
d. lead
e. platinum
f. gold
· 100.
Which of the following series of elements is not matched with the correct description?
a. , , —halogens
b. , , —noble gases
c. , , —alkaline earth metals
d. , , —transition metals
e. , , —metals
· 101.
Give the chemical symbol for each of the following elements.
a. silver
b. aluminum
c. cadmium
d. antimony
e. tin
f. arsenic
· 102.
A metal ion with a charge contains neutrons and electrons. Identify the metal ion and determine its mass number.
· 103.
For each of the following chemical symbols, give the name of the corresponding element.
a.
b.
c.
d.
e.
f.
g.
h.
· 104.
Write the simplest formula for each of the following substances, listing the elements in the order given.
a. a molecule containing one carbon atom and two oxygen atoms
b. a compound containing one aluminum atom for every three chlorine atoms
c. perchloric acid, which contains one hydrogen atom, one chlorine atom, and four oxygen atoms
d. a molecule containing one sulfur atom and six chlorine atoms
· 105.
For each of the following atomic numbers, write the name and chemical symbol of the corresponding element. (Refer to Figure 4.9.)
a.
b.
c.
d.
e.
f.
g.
h.
· 106.
Write the atomic symbol for each of the isotopes described below.
a. ,
b. the isotope of carbon with a mass number of
c. ,
d. ,
e. the isotope of calcium with a mass number of
f. the isotope with protons and neutrons
· 107.
How many protons and neutrons are contained in the nucleus of each of the following atoms? In an atom of each element, how many electrons are present?
a.
b.
c.
d.
e.
f.
· 108.
Complete the following table.
Symbol |
Protons |
Neutrons |
Mass Number |
· 109.
For each of the following elements, use the table shown in Fig. 4.9 to give the chemical symbol and atomic number and to specify whether the element is a metal or a nonmetal. Also give the named family to which the element belongs (if any).
a. carbon
b. selenium
c. radon
d. beryllium
· 110.
![]() Read the “Chemistry in Focus” segment A Four-Wheel-Drive Nanocar. It discusses carbon and copper atoms. Both atoms have stable isotopes. Example 4.2 had you consider the isotopes of carbon. Copper exists as copper-63 and copper-65. Determine the number of each of the three types of subatomic particles in each of the copper atoms and write their symbols.
Read the “Chemistry in Focus” segment A Four-Wheel-Drive Nanocar. It discusses carbon and copper atoms. Both atoms have stable isotopes. Example 4.2 had you consider the isotopes of carbon. Copper exists as copper-63 and copper-65. Determine the number of each of the three types of subatomic particles in each of the copper atoms and write their symbols.
ChemWork Problems
These multiconcept problems (and additional ones) are found interactively online with the same type of assistance a student would get from an instructor.
· 111.
Provide the name of the element that corresponds to each symbol given in the following table.
Symbol |
Element Name |
· 112.
Provide the symbols for the elements given in the following table.
Element Name |
Symbol |
tin |
|
beryllium |
|
hydrogen |
|
chlorine |
|
radium |
|
xenon |
|
zinc |
|
oxygen |
· 113.
Complete the following table.
Number of Protons |
Number of Neutrons |
Symbol |
· 114.
Complete the following table to predict whether the given atom will gain or lose electrons in forming an ion.
Atom |
Gain (G) or Lose (L) Electrons |
Ion Formed |
· 115.
Using the periodic table, complete the following table.
Atoms |
Number of Protons |
Number of Neutrons |
· 116.
Complete the following table.
Atom/Ion |
Protons |
Neutrons |
Electrons |
· 117.
Which of the following is(are) correct?
a. contains protons and electrons.
b. Rutherford created the cathode-ray tube and was the founder of the charge-to-mass ratio of an electron.
c. An electron is heavier than a proton.
d. The nucleus contains protons, neutrons, and electrons.