Liquid-State Physical Chemistry: Fundamentals, Modeling, and Applications (2013)
Appendix F. Numerical Answers to Selected Problems
3.11 Ar: 129 K, HBr: 467 K.
3.16 Taking α1/α2 = 2, we obtain the following results rendering the Berthelot approximation doubtful.

3.17 Ucoh = 14.3 kJ/mol and Hcoh = Ucoh + RT = 16.8 kJ/mol and
Ucoh = 28.9 kJ/mol and Hcoh = Ucoh + RT = 31.4 kJ/mol.
3.18 The relative contribution is (11/32)α/σ3 = 0.011.
5.3 Z = 1 + exp(−ε/kT), p1 = 0.73, p2 = 0.27, U = ε/[exp(ε/kT) + 1],
CV = [exp(ε/kT) + 1]−2(ε2/kT2)exp(ε/kT).
5.11 Λ(He) ≅ 4.3 Å with ρ−1/3 ≅ 3.7 Å → classical approximation not valid;
Λ(Ar) ≅ 0.30 Å with ρ−1/3 ≅ 4.0 Å → classical approximation valid.
5.21 Assuming deviations less than 5% are allowed, one has 1 + θ/3T < 1.05 or T > 7θ.
6.1 ρrel = 0.984·ρglass/ρFCC = 0.984·0.637/0.741 = 0.85. Fig. 6.3 provides 0.9 < V* < 1.1, hence 0.77 < ρrel < 0.95.
6.3
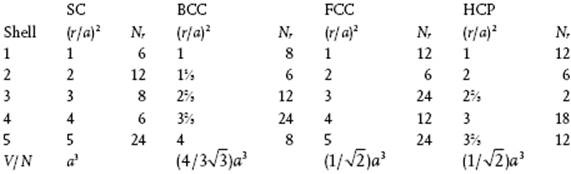
6.4 N(1) = 9.2, N(2) = 44.4.
6.6 a) Nearest neighbour Li-Cl ≅ 2.0 Å, next nearest neighbour Li-Cl ≅ 5.3 Å
b) Nearest neighbour Li-Li ≅ Cl-Cl ≅ 3.7 Å.
7.5 PV/KT = 1 + Σn=1(n2 + 3n)ηn.
7.6 v = 1.70; ![]() ;
; ![]() ;
;
8.2 a) vL/vG = a/(a − d)
b) Vf = (4π/3)(a − d)3 = (4πa3/3)(vG/vL)3 = 0.008 (4πa3/3)
10.3 H2O: εr = 78.5, CH3OH: εr = 31.6, C2H5OH: εr = 24.3.
10.5 α′ = 10.3 Å3, μ = 1.57 D.
10.7 α′ = 6.4 Å3, μ = 2.8 D.
10.10 α′ = 13.8 × 10−30 m3/mol, μ = 0.34 D using the Debye equation. The data point at −110 °C is also at the line for the liquid, indicating that the molecule still rotates in the solid phase. However, α′ is large and μ is small as compared to independent data (α′ = 3.23 × 10−30 m3/mol, μ = 1.7 D) illustrating the effect of hydrogen bonding.
10.11 μ*/μ = 1.26.
11.5 V1 = A + B + C[x1(2 − x1)], ![]() .
.
11.6 ![]() .
.
11.10 a) ΔH = zwx1(1 − x1) = 800 J/mol at x1 = 0.5, hence zw = 3200 and w = 320 J/mol.
b) ![]() or γ2 = 1.083.
or γ2 = 1.083.
11.12 Since the expressions are symmetric in x1 and x2, yes.
12.2 ΔsolH = 4 kJ mol−1 > 0, hence ΔT > 0. TΔS > 4 kJ mol−1 or ΔS > 13.3 J K−1 mol−1.
12.3 ![]() .
.
12.9 Assuming the same structure as in Fig. 12.4, the angle is 40°.
12.11 ΔH = A − DT2, ΔS = C−2DT, ΔCP = −2DT.
12.13 κ−1 ≅ 1.8 nm.
12.17 tH = 3 · 10−7 s.
12.18 a) ΔT = 0.31 °C; b) Λm = 0.0273 Ω−1 mol−1 m2; c) I = 14 mA; d) I = 20 mA using η(H2O) = 1 · 10−3 Pa s, a(Ca2+) = 1.0 Å and a(I−) = 2.15 Å; e) t(Ca2+) = 0.44; f) 2.4 K/hour.
13.1
|
A: L = 10 nm, X = 1.0 nm |
B: L = 100 nm, X = 3.16 nm |
|
C: L = 100 nm, X = 5.47 nm |
D: L = 100 nm, X = 13.2 nm |
13.9 δ = 18.2 MPa1/2.
13.10 δ = 18.3 MPa1/2.
13.11 ϕ1 = 0.10, ϕ2 = 0.41, ϕ3 = 0.49.
15.1 u(σ) = 26.1 mJ m−2.
15.8 γ = 0.0264 N/m. The reasonable good agreement for this calculation of cyclohexane with the experimental value 0.0247 N/m is probably fortuitous.