Liquid-State Physical Chemistry: Fundamentals, Modeling, and Applications (2013)
10. Describing the Behavior of Liquids: Polar Liquids
10.2. Towards a Microscopic Interpretation
So, an applied field E introduces into a material a polarization P = ε0(εr − 1)E., and our task is now to interpret the polarization P in microscopic terms. In general, we have several molecular contributions to the polarization. First, if the molecules are polar and have a dipole moment3) μ, we have the alignment of these permanent dipoles due to the applied field. This process results in a polarization contribution labeled Pμ. Second, even if we have nonpolar molecules, all molecules have an electronic polarizability4) αele and molecular polarizability αmol, collectively denoted by α, that results in a dipole moment induced by the applied field. This contribution is labeled Pα. The field experienced by the molecules is not equal to the applied field E as the surrounding molecules shield the reference molecule from the applied field, and a polar reference molecule will influence the surroundings leading to a different shielding than for a nonpolar molecule. If we have N molecules in a volume V, we may write Pμ = (N/V)pμ and Pα = (N/V)pα. Here, pμ represents the (rotational) average dipole moment ![]() , which is dependent on the directional fieldEdir experienced by the molecules. For pα we have pα = αEint, where Eint is the internal field. Although both fields Edir and Eint are proportional to the applied field E, they are not the same because a proportional increase in all three components of E will affect the magnitude of the induced dipole moment but not the directional effect for the permanent dipole. Hence, our task is to calculate Eint, Edir and
, which is dependent on the directional fieldEdir experienced by the molecules. For pα we have pα = αEint, where Eint is the internal field. Although both fields Edir and Eint are proportional to the applied field E, they are not the same because a proportional increase in all three components of E will affect the magnitude of the induced dipole moment but not the directional effect for the permanent dipole. Hence, our task is to calculate Eint, Edir and ![]() so that Pμ and Pα can be determined.
so that Pμ and Pα can be determined.
Altogether, in microscopic terms polarization can be interpreted as the number of molecules N with (effective) dipole moment μeff in a volume V, that is
(10.9) ![]()
Overall, the effective dipole moment is described by μeff = αeffEloc, where αeff is the effective polarizability of the molecule and Eloc is the local field, the latter being the appropriate combination of the internal and directional field. We deal first with gases and thereafter with liquids and solutions.
Problem 10.3
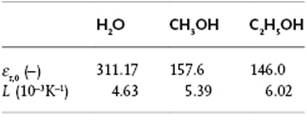
The relative permittivity εr can be accurately described by εr(T) = εr,0exp(−LT), with as parameters εr,0 and L. Calculate εr(298) for the three liquids indicated (see Table 10.1).
Table 10.1 Parameters εr,0 and L for H2O, CH3OH, and C2H5OH.