MCAT General Chemistry Review
Chapter 2: The Periodic Table
2.1 The Periodic Table
In 1869, the Russian chemist Dmitri Mendeleev published the first version of his Periodic Table of the Elements, which showed that ordering the known elements according to atomic weight revealed a pattern of periodically recurring physical and chemical properties. Since then, the Periodic Table has been revised, using the work of physicist Henry Moseley, to organize the elements based on increasing atomic number (the number of protons in an element) rather than atomic weight. Using this revised table, many properties of elements that had not yet been discovered could be predicted. The Periodic Table creates a visual representation of the periodic law, which states: the chemical and physical properties of the elements are dependent, in a periodic way, upon their atomic numbers.
The modern Periodic Table arranges the elements into periods (rows) and groups or families (columns), based on atomic number. There are seven periods, representing the principal quantum numbers n = 1 through n = 7 for the s- and p-block elements. Each period is filled sequentially, and each element in a given period has one more proton and one more electron than the element to its left (in their neutral states). Groups contain elements that have the same electronic configuration in their valence shell and share similar chemical properties.
BRIDGE
Recall from Chapter 1 of MCAT General Chemistry Review that periods (rows) graphically represent the principal quantum number, and groups (columns) help to determine the valence electron configuration.
The electrons in the valence shell, known as the valence electrons, are the farthest from the nucleus and have the greatest amount of potential energy. Their higher potential energy and the fact that they are held less tightly by the nucleus allows them to become involved in chemical bonds with the valence electrons of other atoms; thus, the valence shell electrons largely determine the chemical reactivity and properties of the element.
MCAT EXPERTISE
Relating valence electrons to reactivity is important. Elements with similar valence electron configurations generally behave in similar ways, as long as they are the same type (metal, nonmetal, or metalloid).
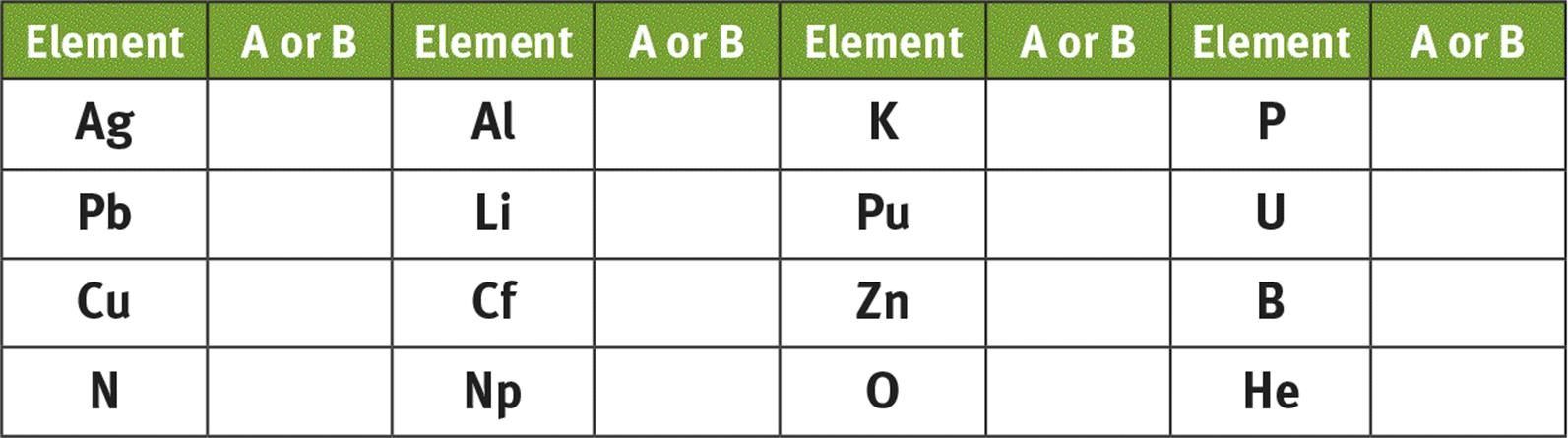
The Roman numeral above each group represents the number of valence electrons elements in that group have in their neutral state. The Roman numeral is combined with the letter A or B to separate the elements intotwo larger classes. The A elements are known as the representative elements and include groups IA through VIIIA. The elements in these groups have their valence electrons in the orbitals of either s or p subshells. The B elements are known as the nonrepresentative elements and include both the transition elements, which have valence electrons in the sand d subshells, and the lanthanide and actinide series, which have valence electrons in the s and f subshells. For therepresentative elements, the Roman numeral and the letter designation determine the electron configuration. For example, an element in Group VA has five valence electrons with the configuration s2p3. As described in Chapter 1 of MCAT General Chemistry Review, the nonrepresentative elements may have unexpected electron configurations, such as chromium (4s1 3d 5) and copper (4s1 3d10). In the modern IUPAC identification system, the groups are numbered 1 to 18 and are not subdivided into Group A and Group B elements.
MCAT Concept Check 2.1:
Before you move on, assess your understanding of the material with these questions.
1. Mendeleev’s table was arranged by atomic weight, but the modern Periodic Table is arranged by:
2. Which of the following are representative elements (A), and which are nonrepresentative (B)?