MCAT General Chemistry Review
Chapter 3: Bonding and Chemical Interactions
3.3 Covalent Bonds
When two or more atoms with similar electronegativities interact, the energy required to form ions through the complete transfer of one or more electrons is greater than the energy that would be released upon the formation of an ionic bond. That is, when two atoms of similar tendency to attract electrons form a compound, it is energetically unfavorable to create ions. Rather than transferring electrons to form octets, the atoms share electrons. The binding force between the atoms is not ionic; instead, there is an attraction that each electron in the shared pair has for the two positive nuclei of the bonded atoms.
MCAT EXPERTISE
Think of bonds as a tug-of-war between two atoms. When the difference in electronegativity is great (more than 1.7), then the “stronger” molecule wins all of the electrons and becomes the anion. When the electronegativity values are relatively similar, then we have a stalemate, or a covalent bond with mostly equal sharing of electrons.
Covalent compounds contain discrete molecular units with relatively weak intermolecular interactions. As a result, compounds like carbon dioxide (CO2) tend to have lower melting and boiling points. In addition, because they do not break down into constituent ions, they are poor conductors of electricity in the liquid state or in aqueous solutions.
PROPERTIES OF COVALENT COMPOUNDS
The formation of one covalent bond may not be sufficient to fill the valence shell for a given atom. Thus, many atoms can form bonds with more than one other atom, and most atoms can form multiple bonds with other atoms. Two atoms sharing one, two, or three pairs of electrons are said to be joined by a single, double, or triple covalent bond, respectively. The number of shared electron pairs between two atoms is called the bond order; hence, a single bond has a bond order of one, a double bond has a bond order of two, and a triple bond has a bond order of three. Three features characterize a covalent bond: bond length, bond energy, and polarity.
Bond Length
Bond length is the average distance between the two nuclei of atoms in a bond. As the number of shared electron pairs increases, the two atoms are pulled closer together, resulting in a decrease in bond length. Thus, for a given pair of atoms, a triple bond is shorter than a double bond, which is shorter than a single bond.
Bond Energy
Bond energy is the energy required to break a bond by separating its components into their isolated, gaseous atomic states. The greater the number of pairs of electrons shared between the atomic nuclei, the more energy is required to break the bonds holding the atoms together. Thus, triple bonds have the greatest bond energy, and single bonds have the lowest bond energy. We will discuss bond energy and calculations involving bond enthalpy in Chapter 7 of MCAT General Chemistry Review. By convention, the greater the bond energy is, the stronger the bond.
KEY CONCEPT
You will see this inverse relationship between bond length and strength in both organic and general chemistry.
|
Bond Length |
Bond Strength |
|
|
C–C |
longest |
weakest |
|
C=C |
medium |
medium |
|
C≡C |
shortest |
strongest |
Know this relationship on Test Day and you’ll earn quick points!
Polarity
Polarity occurs when two atoms have a relative difference in electronegativities. When these atoms come together in covalent bonds, they must negotiate the degree to which the electron pairs will be shared. The atom with the higher electronegativity gets the larger share of the electron density. A polar bond creates a dipole, with the positive end of the dipole at the less electronegative atom and the negative end at the more electronegative atom, as shown in Figure 3.5.
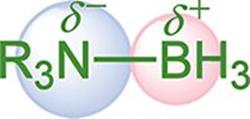 Figure 3.5. Polar Covalent Bond in an Amine Borane Nitrogen takes on a partial negative charge (δ–), boron takes on a partial positive charge (δ+).
Figure 3.5. Polar Covalent Bond in an Amine Borane Nitrogen takes on a partial negative charge (δ–), boron takes on a partial positive charge (δ+).
When atoms that have identical or nearly identical electronegativities share electron pairs, they do so with equal distribution of the electrons. This is called a nonpolar covalent bond, and there is no separation of charge across the bond. Note that only bonds between atoms of the same element will have exactly the same electronegativity and therefore exhibit a purely equal distribution of electrons. The seven common diatomic molecules are H2, N2, O2, F2, Cl2, Br2, and I2. At the same time, many bonds are close to nonpolar. Any bond between atoms with a difference in electro-negativity less than 0.5 is generally considered nonpolar.
MNEMONIC
Here’s a quick way to remember the naturally occurring diatomic elements on the Periodic Table: they form the number 7 on the Periodic Table (except for H), there are 7 of them, and most of them are in Group VIIA: H2, N2, O2, F2, Cl2, Br2, I2.
Polar Covalent Bond
Atoms that differ moderately in their electronegativities will share electrons unevenly, resulting in polar covalent bonds. While the difference in their electronegativities (between 0.5 and 1.7) is not enough to result in the formation of an ionic bond, it is sufficient to cause a separation of charge across the bond. This results in the more electronegative element acquiring a greater portion of the electron density, taking on a partial negative charge (δ–), and the less electronegative element acquiring a smaller portion of the electron density, taking on a partial positive charge (δ+). For instance, the covalent bond in HCl is polar because the two atoms have a moderate difference in electronegativity (ΔEN = 0.9). In this bond, the chlorine atom gains a partial negative charge, and the hydrogen atom gains a partial positive charge. The difference in charge between the atoms is indicated by an arrow crossed at its tail end (giving the appearance of a “plus” sign) and pointing toward the negative end, as shown in Figure 3.6.
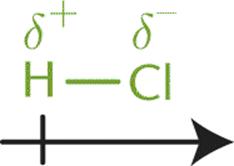 Figure 3.6. Dipole Moment of HCl
Figure 3.6. Dipole Moment of HCl
MCAT EXPERTISE
The range of electronegativities for nonpolar bonds is roughly 0 to 0.5. Polar bonds are found from 0.5 to 1.7, and ionic bonds are at 1.7 and above. Some chemistry courses allude to a grey area from 1.7 to 2.0. For the MCAT, if a molecule in this range has a metal and nonmetal, then it is effectively ionic; otherwise, it is polar covalent.
A molecule that has such a separation of positive and negative charges is called a polar molecule. The dipole moment of the polar bond or polar molecule is a vector quantity given by the equation:
p = qd
Equation 3.1
where p is the dipole moment, q is the magnitude of the charge, and d is the displacement vector separating the two partial charges. The dipole moment vector, represented by an arrow pointing from the positive to the negative charge, is measured in Debye units (coulomb–meters).
COORDINATE COVALENT BONDS
In a coordinate covalent bond, both of the shared electrons originated on the same atom. Generally, this means that a lone pair of one atom attacked another atom with an unhybridized p-orbital to form a bond, as shown in Figure 3.7. Once such a bond forms, however, it is indistinguishable from any other covalent bond. The distinction is only helpful for keeping track of the valence electrons and formal charges. Coordinate covalent bonds are typically found in Lewis acid–base reactions, described in Chapter 10 of MCAT General Chemistry Review. A Lewis acid is any compound that will accept a lone pair of electrons, while a Lewis base is any compound that will donate a pair of electrons to form a covalent bond.
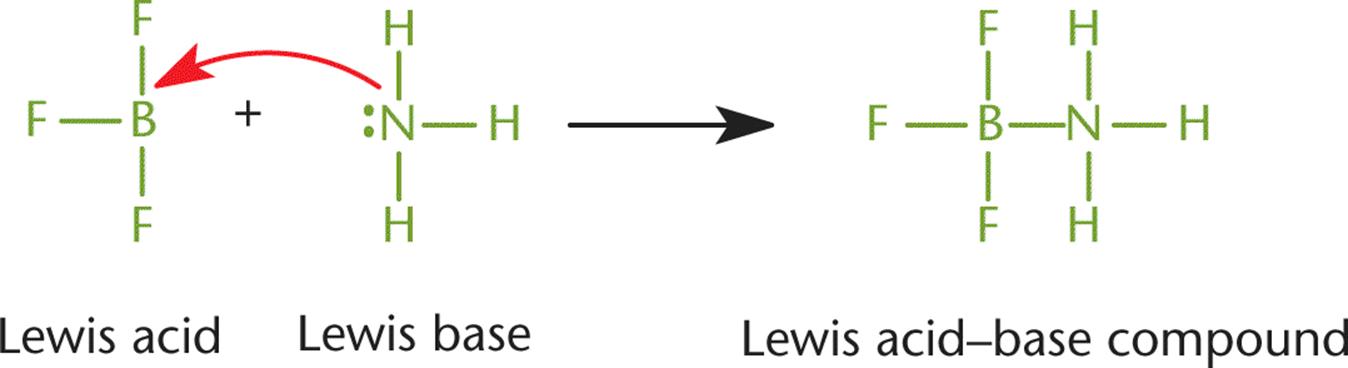 Figure 3.7. Coordinate Covalent Bond
Figure 3.7. Coordinate Covalent Bond
BRIDGE
The chemistry that creates coordinate covalent bonds appears in many guises. These reactions can be called nucleophile–electrophile reactions, described in Chapter 4 of MCAT Organic Chemistry Review; Lewis acid–base reactions, described in Chapter 10 of MCAT General Chemistry Review, or complexation reactions, described in Chapter 9 of MCAT General Chemistry Review.
Here, NH3 donates a pair of electrons to form a coordinate covalent bond; thus, it acts as a Lewis base. At the same time, BF3 accepts this pair of electrons to form the coordinate covalent bond; thus, it acts as a Lewis acid.
COVALENT BOND NOTATION
The electrons involved in a covalent bond are in the valence shell and are bonding electrons, while those electrons in the valence shell that are not involved in covalent bonds are nonbonding electrons. The unshared electron pairs are also known as lone pairs because they are associated only with one atomic nucleus. Because atoms can bond with other atoms in many different combinations, the Lewis structure system of notation was developed to keep track of the bonded and nonbonded electron pairs.
Think of Lewis structures as a bookkeeping method for electrons. The number of valence electrons attributed to a particular atom in the Lewis structure of a molecule is not necessarily the same as the number of valence electrons in the neutral atom. This difference accounts for the formal charge of an atom in a Lewis structure. Often, more than one Lewis structure can be drawn for a molecule. If the possible Lewis structures differ in their bond connectivity or arrangement, then the Lewis structures represent different possible compounds. However, if the Lewis structures show the same bond connectivity and differ only in the arrangement of the electron pairs, then these structures represent different resonance forms of a single compound. Note that Lewis structures do not represent the actual or even theoretical geometry of a real compound. Their usefulness lies in showing the different possible ways in which atoms may be combined to form different compounds or resonance forms of a single compound.
When more than one arrangement can be made, one can assess the likelihood of each arrangement by checking the formal charges on the atoms in each arrangement. The arrangement that minimizes the number and magnitude of formal charges is usually the most stable arrangement of the compound.
Lewis Structures
A Lewis structure, or Lewis dot diagram, is the chemical symbol of an element surrounded by dots, each representing one of the s or p valence electrons of the atom. The Lewis symbols of the elements in the second period of the Periodic Table are shown in Table 3.1.
|
|
Lithium |
|
Nitrogen |
|
|
Berylliu |
|
Oxygen |
|
|
Boron |
|
Fluorine |
|
|
Carbon |
|
Neon |
|
Table 3.1. Lewis Symbols for Period 2 Elements |
|||
KEY CONCEPT
In drawing Lewis dot structures, remember that some atoms can expand their octets by utilizing the d-orbitals in their outer shell. This will only take place with atoms in period 3 or greater.
MCAT EXPERTISE
The number of dots in Lewis Structure notation comes from group numbers. Lithium is in Group IA and therefore has one electron (dot). Carbon is in Group IVA and has four dots.
Just as a Lewis symbol is used to represent the distribution of valence electrons in an atom, it can also be used to represent the distribution of valence electrons in a molecule. For example, the Lewis symbol for a fluoride ion, F–, is 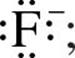 the Lewis structure of the diatomic molecule F2 is
the Lewis structure of the diatomic molecule F2 is 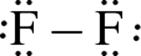 . Certain rules must be followed in assigning a Lewis structure to a molecule. The steps for drawing a Lewis structure are outlined here, using HCN as an example.
. Certain rules must be followed in assigning a Lewis structure to a molecule. The steps for drawing a Lewis structure are outlined here, using HCN as an example.
· Draw out the backbone of the compound—that is, the arrangement of atoms. In general, the least electronegative atom is the central atom. Hydrogen (always) and the halogens F, Cl, Br, and I (usually) occupy a terminal position.
In HCN, H must occupy an end position. Of the remaining two atoms, C is the least electronegative and, therefore, occupies the central position. Therefore, the skeletal structure is as follows:
H – C – N
· Count all the valence electrons of the atoms. The number of valence electrons of the molecule is the sum of the valence electrons of all atoms present:
· H has 1 valence electron
· C has 4 valence electrons
· N has 5 valence electrons; therefore,
· HCN has a total of 10 valence electrons.
· Draw single bonds between the central atom and the atoms surrounding it. Each single bond corresponds to a pair of electrons:
H : C : N
· Complete the octets of all atoms bonded to the central atom, using the remaining valence electrons left be assigned. Recall that H is an exception to the octet rule because it can only have two valence electrons. In this example, H already has two valence electrons from its bond with C.
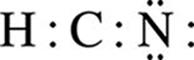
· Place any extra electrons on the central atom. If the central atom has less than an octet, try to write double or triple bonds between the central and surrounding atoms using the lone pairs on the surrounding atoms.
The HCN structure above does not satisfy the octet rule for C because C only has four valence electrons. Therefore, two lone electron pairs from the N atom must be moved to form two more bonds with C, creating a triple bond between C and N. To make it easier to visualize, bonding electron pairs are represented as lines. You should be familiar with both dot and line notation for bonds.
H – C ≡ N:
Now, the octet rule is satisfied for all three atoms; C and N have eight valence electrons, and H has two valence electrons.
Formal Charge
To determine if a Lewis structure is representative of the actual arrangement of atoms in a compound, one must calculate the formal charge of each atom. In doing so, assume a perfectly equal sharing of all bonded electron pairs, regardless of actual differences in electronegativity. In other words, assume that each electron pair is split evenly between the two nuclei in the bond. The difference between the number of electrons assigned to an atom in a Lewis structure and the number of electrons normally found in that atom’s valence shell is the formal charge. A simple equation you can use to calculate formal charge is:
![]()
Equation 3.2
where V is the normal number of electrons in the atom’s valence shell, Nnonbonding is the number of nonbonding electrons, and Nbonding is the number of bonding electrons (double the number of bonds because each bond has two electrons). The charge of an ion or compound is equal to the sum of the formal charges of the individual atoms comprising the ion or compound.
Example:
Calculate the formal charge on the central N atom of [NH4]+.
Solution:
The Lewis structure of [NH4]+ is:
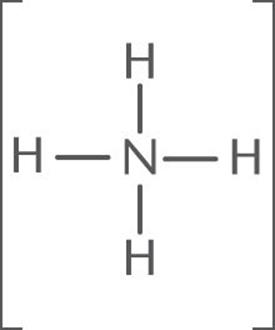
Nitrogen is in group VA; thus it has five valence electrons. In [NH4]+, N has 4 bonds (eight bonding electrons and zero nonbonding electrons).
· Thus, V = 5; Nbonding = 8; Nnonbonding = 0
· ![]()
· Thus, the formal charge on the N atom in [NH4]+ is +1.
One can also use logic to determine formal charge. As drawn, N has four bonds. Assuming equal sharing of the electrons in the bonds, this means N has four valence electrons. In its normal state, N has five valence electrons. Thus, nitrogen has one fewer electron that its normal state, and has a +1 charge.
Let us offer a brief note of explanation on the difference between formal charge and oxidation number: formal charge underestimates the effect of electronegativity differences, whereas oxidation numbers overestimate the effect of electro-negativity differences, assuming that the more electronegative atom has a 100 percent share of the bonding electron pair. For example, in a molecule of CO2 (carbon dioxide), the formal charge on each of the atoms is 0, but the oxidation number of each of the oxygen atoms is −2 and of the carbon is +4. In reality, the distribution of electron density between the carbon and oxygen atoms lies somewhere between the extremes predicted by the formal charges and the oxidation states.
Resonance
As suggested earlier, it may be possible to draw two or more Lewis structures that demonstrate the same arrangement of atoms but that differ in the specific placement of the electrons. These are called resonance structures and are represented with a double-headed arrow between them. The actual electronic distribution in the compound is a hybrid, or composite, of all of the possible resonance structures. For example, SO2 has three resonance structures, as shown in Figure 3.8.
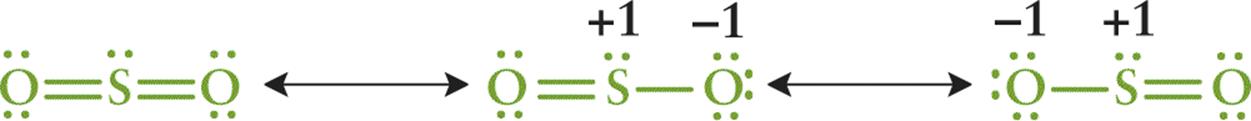 Figure 3.8 Resonance Structures for SO2 The double-headed arrows indicate that these molecules are involved in a resonance hybrid.
Figure 3.8 Resonance Structures for SO2 The double-headed arrows indicate that these molecules are involved in a resonance hybrid.
BRIDGE
Resonance is an important topic in both general and organic chemistry. It allows for greater stability, delocalizing electrons and charges over what is known as a π (pi) system. Resonance in organic molecules is discussed in Chapter 3 of MCAT Organic Chemistry Review.
The nature of the bonds within the actual compound is a hybrid of these three structures. If one were to evaluate the spectral data, it would indicate that the two S–O bonds are identical and equivalent. This phenomenon is known as resonance, and the actual structure of the compound is called the resonance hybrid.
The first resonance structure in Figure 3.8 is significantly more stable than the other two structures. Consequently, it is the major contributor to the resonance hybrid. In general, the more stable the structure, the more it contributes to the character of the resonance hybrid. In Figure 3.8, the minor contributors contain formal charges, indicating decreased stability. One can use formal charge to assess the stability of resonance structures according to the following guidelines:
· A Lewis structure with small or no formal charges is preferred over a Lewis structure with large formal charges
· A Lewis structure with less separation between opposite charges is preferred over a Lewis structure with a large separation of opposite charges
· A Lewis structure in which negative formal charges are placed on more electronegative atoms is more stable than one in which the negative formal charges are placed on less electronegative atoms
Example:
Write the resonance structures for [NCO]–.
Solution:
1. C is the least electronegative of the three given atoms. Therefore, the C atom occupies the central position in the skeletal structure of [NCO]–:
N – C – O
2.
o N has 5 valence electrons;
o C has 4 valence electrons;
o O has 6 valence electrons;
o and the species has one negative charge.
o Total valence electrons = 5 + 4 + 6 + 1 = 16
3. Draw single bonds between the central C atom and the surrounding atoms, N and O. Draw a pair of electrons to represent each bond.
N : C : O
4. Complete the octets of N and O with the remaining 12 electrons.

5. The C octet is incomplete. There are three ways in which double and triple bonds can be formed to complete the C octet: two lone pairs from the O atom can be used to form a triple bond between the C and O atoms:
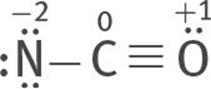
Or one lone electron pair can be taken from both O and N to form two double bonds, one between N and C, the other between O and C:
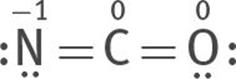
Or two lone electron pairs can be taken from the N atom to form a triple bond between the C and N atoms:
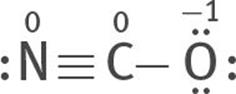
All three are resonance structures of [NCO]–
6. Assign formal charges to each atom of each resonance structure.
The most stable structure is this:
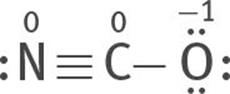
because the charges are minimized, and the negative formal charge is on the most electronegative atom, O.
Exceptions to the Octet Rule
As stated previously, the octet rule has many exceptions. In addition to hydrogen, helium, lithium, beryllium, and boron, which are exceptions because they cannot or do not reach the octet, all elements in or beyond the third period may be exceptions because they can take on more than eight electrons in their valence shells. These electrons can be placed into orbitals of the d subshell, and as a result, atoms of these elements can form more than four bonds. On Test Day, don’t automatically discount a Lewis structure with a central atom that has more than four bonds—the testmakers may be testing your ability to recognize that many atoms can expand their valence shells beyond the octet.
Consider the sulfate ion, SO42−. In the Lewis structure for the sulfate ion, giving the sulfur 12 valence electrons permits three of the five atoms to be assigned a formal charge of zero. The sulfate ion can be drawn in at least six resonance forms, many of which have two double bonds attached to a different combination of oxygen atoms. Figure 3.9 shows two of the possible forms.
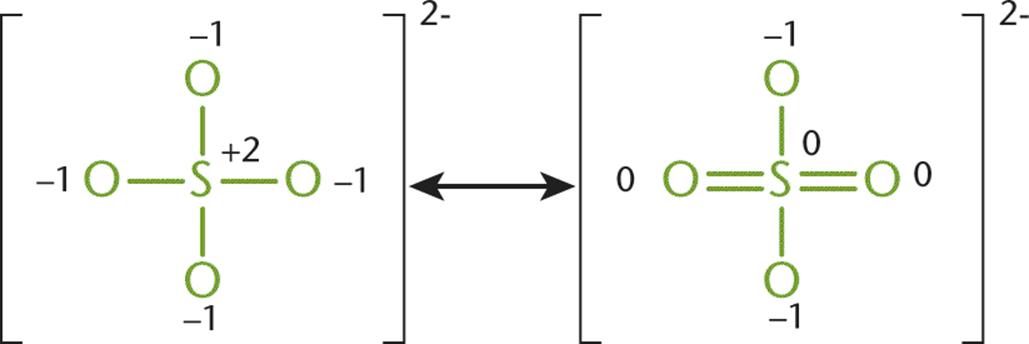 Figure 3.9. Two Different Resonance Forms of the Sulfate Ion
Figure 3.9. Two Different Resonance Forms of the Sulfate Ion
MCAT EXPERTISE
Don’t be surprised if you can draw more than two resonance structures for a molecule or ion. Becoming proficient at drawing and, more importantly, recognizing resonance structures will save you time on Test Day.
GEOMETRY AND POLARITY
Because Lewis dot structures do not suggest or reflect the actual geometric arrangement of atoms in a compound, we need another system to provide this information. One such system is known as the valence shell electron pair repulsion (VSEPR) theory.
Valence Shell Electron Pair Repulsion (VSEPR) Theory
VSEPR theory uses Lewis dot structures to predict the molecular geometry of covalently bonded molecules. It states that the three-dimensional arrangement of atoms surrounding a central atom is determined by the repulsions between bonding and nonbonding electron pairs in the valence shell of the central atom. These electron pairs arrange themselves as far apart as possible, thereby minimizing repulsive forces. The following steps are used to predict the geometrical structure of a molecule using the VSEPR theory:
· Draw the Lewis dot structure of the molecule
· Count the total number of bonding and nonbonding electron pairs in the valence shell of the central atom
· Arrange the electron pairs around the central atom so that they are as far apart as possible. For example, the compound AX2 has the Lewis structure X : A : X. The A atom has two bonding electron pairs in its valence shell. To position these electron pairs as far apart as possible, their geometric structure should be linear:
X – A – X
A summary of molecular geometries as predicted by VSEPR theory is shown in Table 3.2.
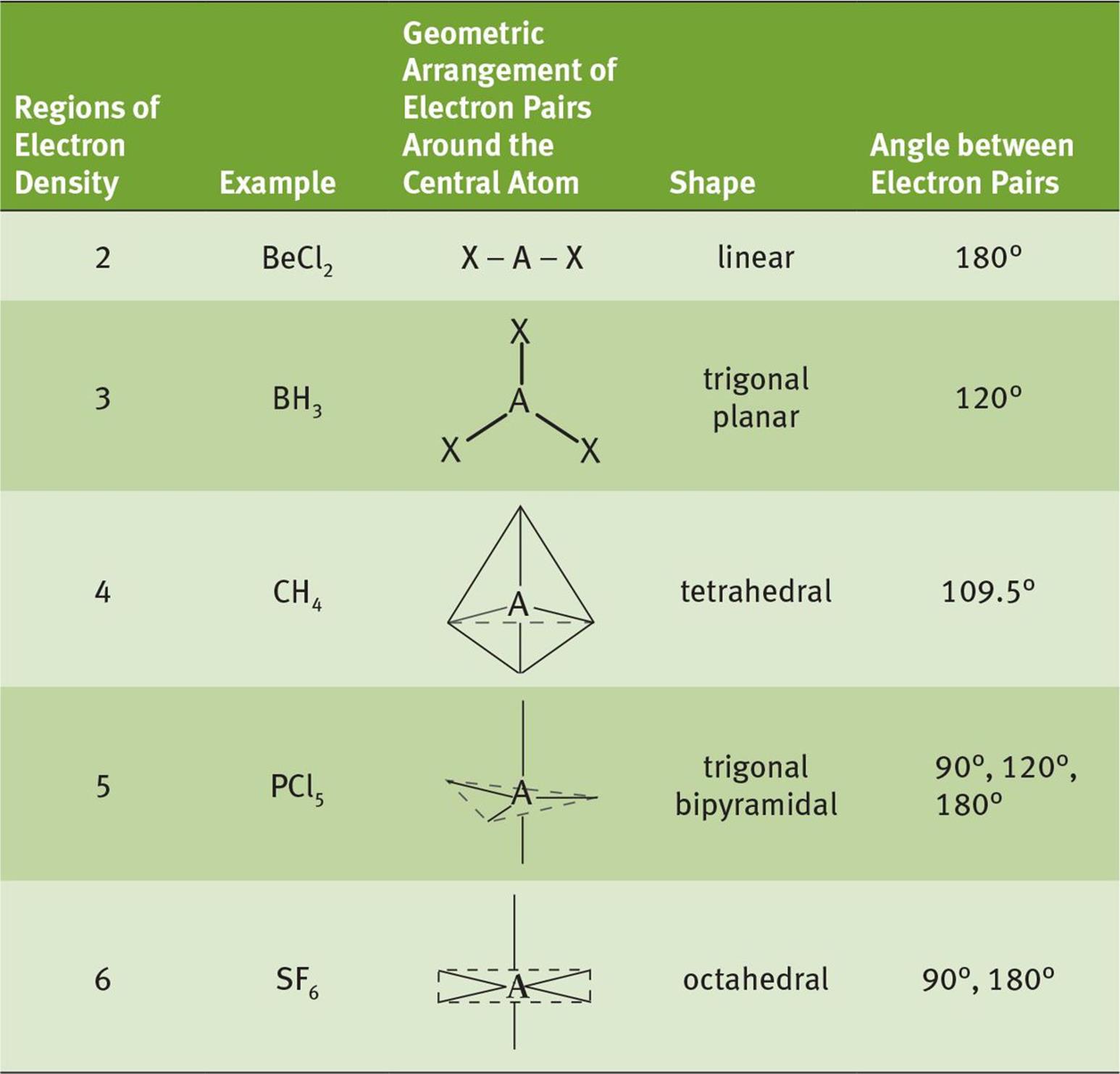
Table 3.2. VSEPR Theory
This table lists the five most common configurations of molecules that do not have a charge or lone pair of electrons around the central atom.
Example:
Predict the molecular geometry of NH3.
Solution:
1. The Lewis structure of NH3 is:
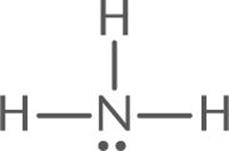
2. The central atom, N, has three bonding electron pairs and one nonbonding electron pair, for a total of four electron pairs.
3. The four electron pairs will be farthest apart when they occupy the corners of a tetrahedron. Because one of the four electron pairs is a lone pair, the observed geometry is trigonal pyramidal, shown below.
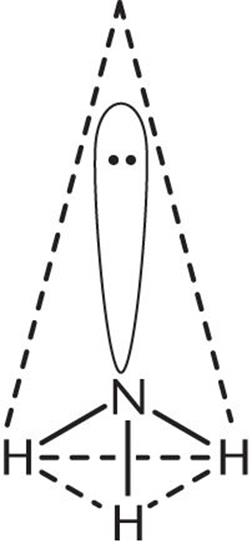
In describing the shape of a molecule, only the arrangement of atoms (not electrons) is considered. Even though the electron pairs are arranged tetrahedrally, the shape of NH3 is pyramidal. It is not trigonal planar because the lone pair repels the three bonding electron pairs, causing them to move as far apart as possible.
Example:
Predict the geometry of CO2.
Solution:
The Lewis structure of CO2 is 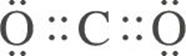
The double bond behaves just like a single bond for the purposes of predicting molecular shape. This compound has two groups of electrons around the carbon. According to the VSEPR theory, the two sets of electrons will orient themselves 180° apart, on opposite sides of the carbon atom, minimizing electron repulsion. Therefore, the molecular structure of CO2 is linear: 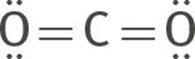
KEY CONCEPT
The shapes from Table 3.2 refer to electronic geometry, which is different from molecular geometry. In the worked example, notice that the ammonia molecule has a tetrahedral electronic structure, but is considered to have a molecular structure that is trigonal pyramidal.
One subtlety that the MCAT loves to test is the difference between electronic geometry and molecular geometry. Electronic geometry describes the spatial arrangement of all pairs of electrons around the central atom, including both the bonding and the lone pairs. In contrast, the molecular geometry describes the spatial arrangement of only the bonding pairs of electrons. For example, consider that CH4 (methane), NH3 (ammonia), and H2O all have the same electronic geometry: in each compound, four pairs of electrons surround the central atom. This is tetrahedral electronic geometry. However, because each molecule has a different number of lone pairs, they have different molecular geometries. Methane has tetrahedral geometry, ammonia is trigonal pyramidal, and water is identified as angular or bent.
MCAT EXPERTISE
CH4, NH3, and H2O all have a tetrahedral electronic geometry, but differ in their molecular shapes:
CH4 is tetrahedral, NH3 is pyramidal, and H2O is bent or angular.
The distinction is important, and the MCAT will primarily focus on molecular geometry. However, there is one important implication of electronic geometry: the determination of the ideal bond angle. Tetrahedral electronic geometry, for example, is associated with an ideal bond angle of 109.5°; however, nonbonding pairs are able to exert more repulsion than bonding pairs because these electrons reside closer to the nucleus. Thus, the angle in ammonia is closer to 107°, and the angle in water is 104.5°.
Polarity of Molecules
When two atoms of different electronegativities bond covalently, sharing one or more pairs of electrons, the resulting bond is polar, with the more electro-negative atom possessing the greater share of the electron density. However, the presence of bond dipoles does not necessarily result in a molecular dipole; that is, an overall separation of charge across the molecule. We must first consider the molecular geometry and the vector addition of the bond dipoles based upon that molecular geometry. A compound with nonpolar bonds is always nonpolar. However, a compound with polar bonds may be polar or nonpolar, depending upon the spatial orientation of the polar bonds in the molecule. If the compound has a molecular geometry such that the bond dipole moments cancel each other out (that is, if the vector sum is zero), then the result is a nonpolar compound. For example, CCl4 (carbon tetrachloride) has four polar C–Cl bonds, but because the molecular geometry of carbon tetrachloride is tetrahedral, the four bond dipoles point to the vertices of the tetrahedron and, therefore, cancel each other out, resulting in a nonpolar compound, as shown in Figure 3.10.
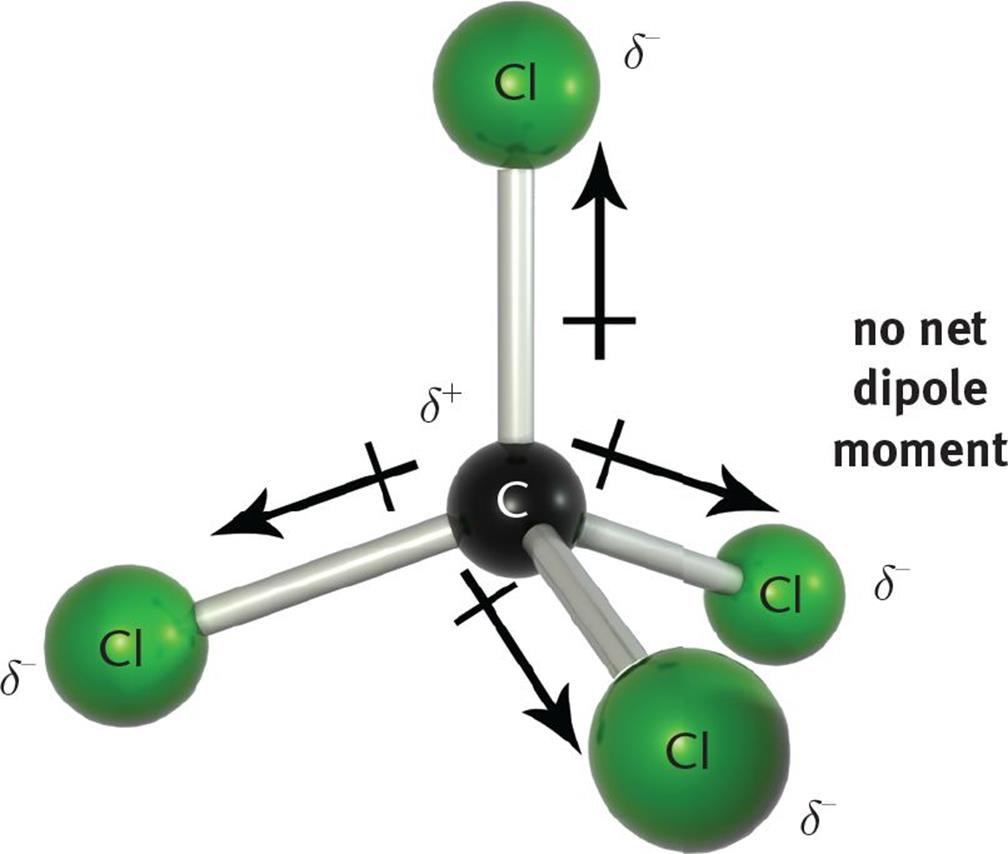 Figure 3.10. CCl4 is a Nonpolar Compound with Four Polar Bonds
Figure 3.10. CCl4 is a Nonpolar Compound with Four Polar Bonds
However, when the molecular geometry is arranged such that the bond dipoles do not cancel each other out, the molecule will have a net dipole moment and will therefore be polar. For instance, the O–H bonds in H2O are polar, with each hydrogen molecule assuming a partial positive charge and the oxygen assuming a partial negative charge. Recall that the molecular geometry of water is angular (bent). Therefore, the vector summation of the bond dipoles results in a molecular dipole moment from the partially positive hydrogen end to the partially negative oxygen end, as illustrated in Figure 3.11.
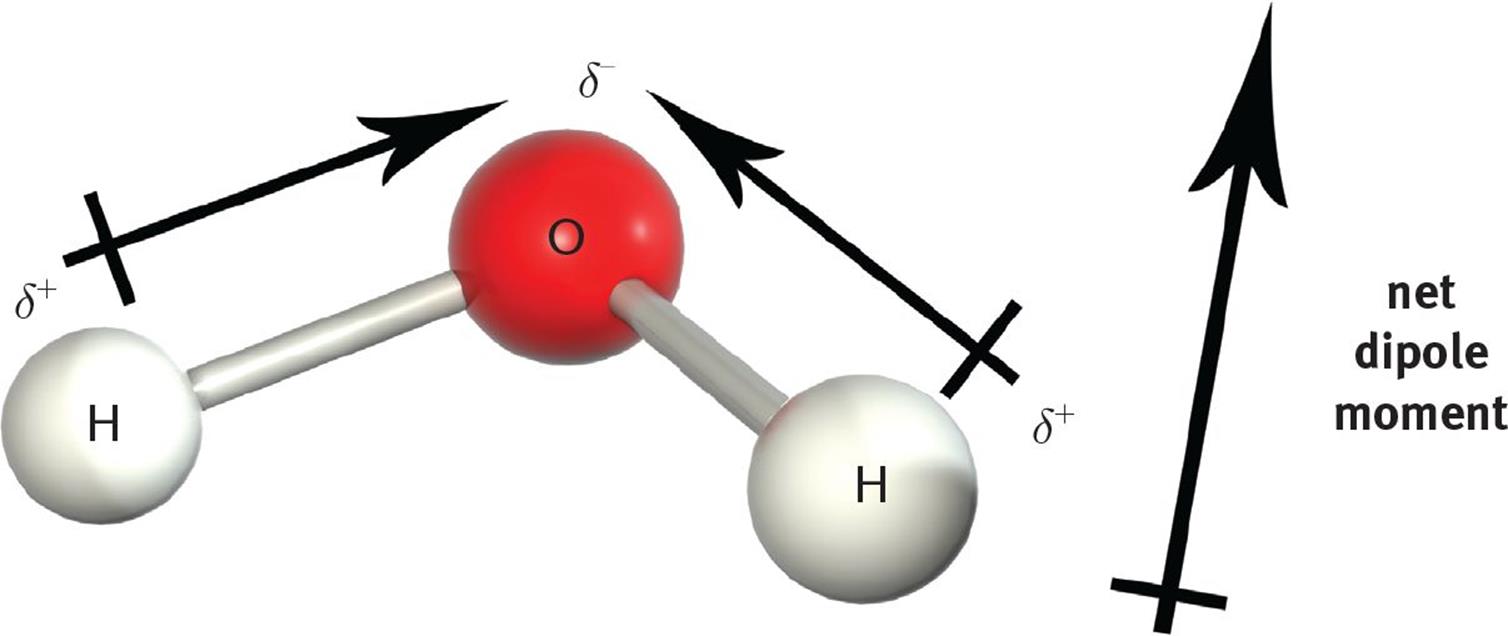 Figure 3.11. H2O is a Polar Molecule with Two Polar Bonds
Figure 3.11. H2O is a Polar Molecule with Two Polar Bonds
MCAT EXPERTISE
Be careful! If you spot a polar bond in a molecule, the molecule can be either polar or nonpolar. On the contrary, when you see only nonpolar bonds in a molecule, the structure must be nonpolar. Always draw out relevant structures on your scratch paper on Test Day.
ATOMIC AND MOLECULAR ORBITALS
To finish the discussion of covalent bonds, we need to address the concept of atomic and molecular orbitals. Recall the model of the atom as a dense, positively charged nucleus surrounded by a cloud of electrons organized into orbitals (regions in space surrounding the nucleus within which there are certain probabilities of finding an electron). The four quantum numbers describe the energy and position of an electron in an atom. While the principal quantum number, n, indicates the average energy level of the orbitals, the azimuthal quantum number, l, describes the subshells within each principal energy level. When l = 0, this indicates the s subshell, which has one orbital that is spherical in shape. The 1s-orbital (n = 1, l = 0, ml = 0) is plotted in Figure 3.12.
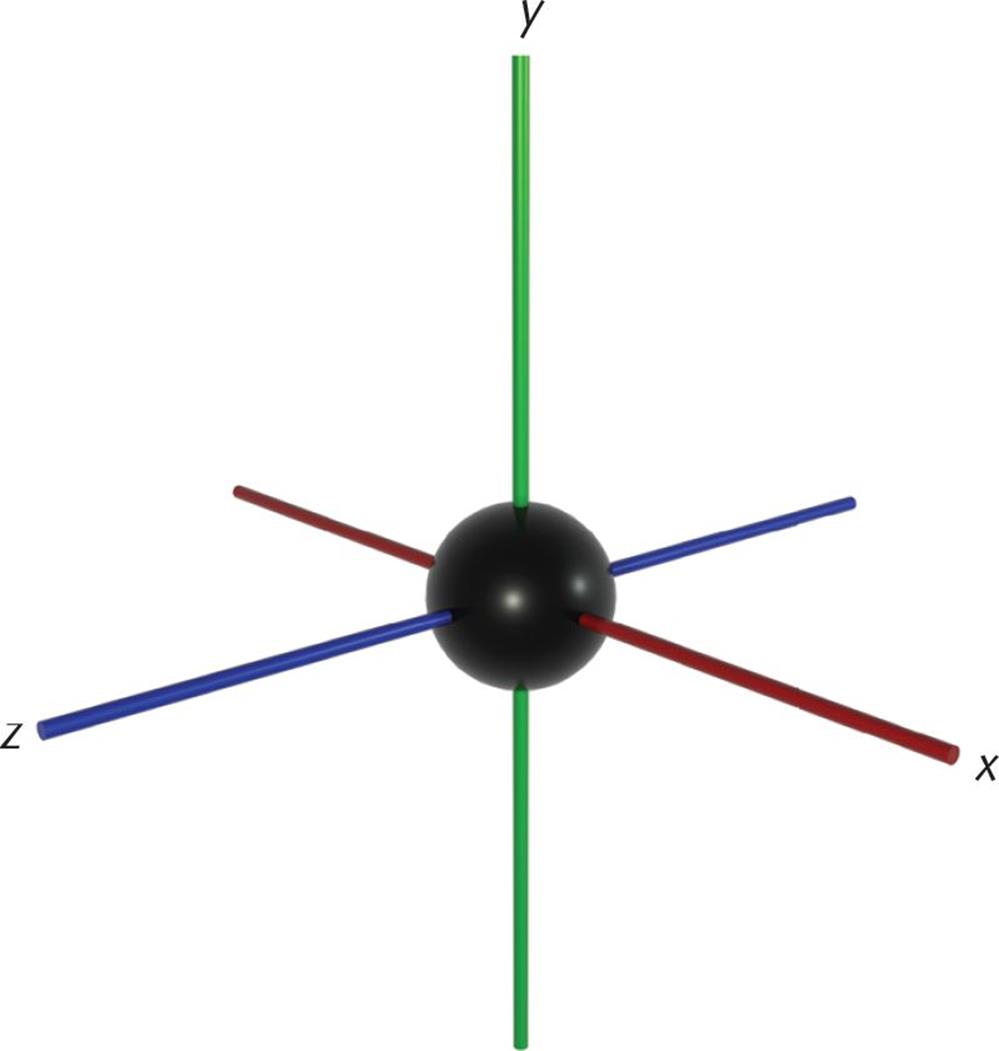 Figure 3.12. 1s-Orbital
Figure 3.12. 1s-Orbital
BRIDGE
Quantum Numbers (Chapter 1 of MCAT General Chemistry Review) revisited:
For any value of n, there are n values of l (0 → n – 1).
· l = 0 → s
· l = 1 → p
· l = 2 → d
· l = 3 → f
For any value of l, there are 2l + 1 values of ml (number of orbitals); values range from –l to l.
When l = 1, this indicates the p subshell, which has three orbitals shaped like barbells along the x-, y-, and z-axes at right angles to each other. The 2p-orbitals (n = 2, l = 1, ml = −1, 0, and +1) are plotted in Figure 3.13.
Although well beyond the scope of the MCAT, mathematical analysis of the wave functions of the orbitals is used to determine and assign plus and minus signs to each lobe of the p-orbitals. The shapes of the five d-orbitals and the seven f-orbitals are more complex and do not need to be memorized for the MCAT.
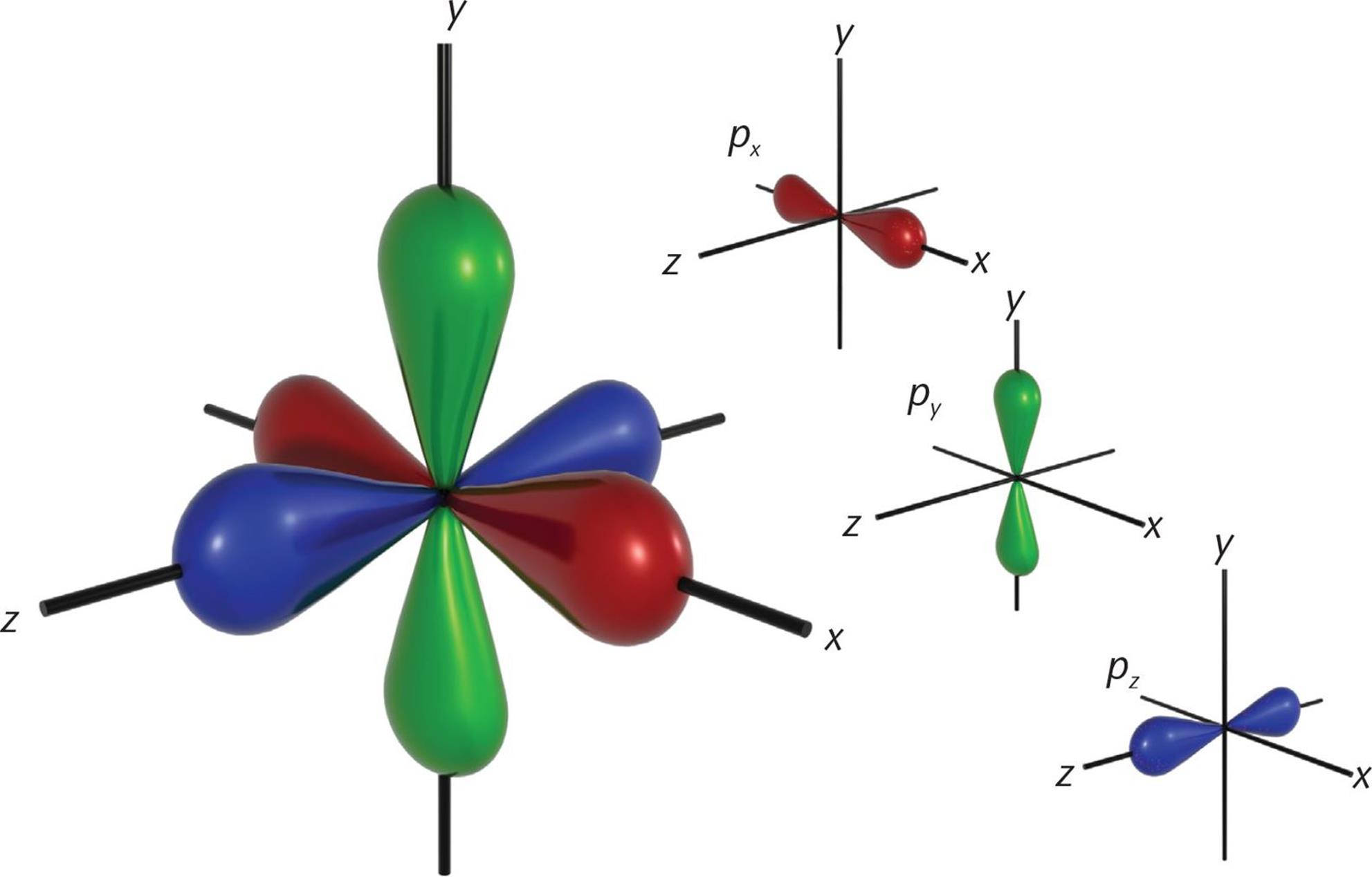 Figure 3.5. p-Orbitals on the x-, y-, and z-Axes
Figure 3.5. p-Orbitals on the x-, y-, and z-Axes
When two atoms bond to form a compound, the atomic orbitals interact to form a molecular orbital that describes the probability of finding the bonding electrons in a given space. Molecular orbitals are obtained by combining the wave functions of the atomic orbitals. Qualitatively, the overlap of two atomic orbitals describes this molecular orbital. If the signs of the two atomic orbitals are the same, a bonding orbital forms. If the signs are different, an antibonding orbital forms.
Two different patterns of overlap are observed in the formation of molecular bonds. When orbitals overlap head-to-head, the resulting bond is a sigma (σ) bond. σ bonds allow for free rotation about their axes because the electron density of the bonding orbital is a single linear accumulation between the atomic nuclei.
When the orbitals overlap in such a way that there are two parallel electron cloud densities, a pi (π) bond is formed. π bonds do not allow for free rotation because the electron densities of the orbitals are parallel and cannot be twisted in such a way that allows continuous overlapping of the clouds of electron densities.
MCAT Concept Check 3.3:
Before you move on, assess your understanding of the material with these questions.
1. Describe the relationship between bond strength, bond length, and bond energy.
2. For what values of ΔEN will a nonpolar covalent bond form? Polar covalent? Ionic?
· Nonpolar covalent:
· Polar covalent:
· Ionic:
3. Draw a Lewis dot structure for the carbonate ion (CO32–) and its two other resonance structures.
4. Predict the molecular geometries of the following molecules:
· PCl5:
· MgF2:
· AlF3:
· UBr6:
· SiH4: