MCAT General Chemistry Review
Chapter 3: Bonding and Chemical Interactions
Conclusion
This chapter built on our knowledge of the atom and the trends demonstrated by the elements in the Periodic Table to explain the different ways by which atoms partner together to form compounds, either by exchanging electrons to form ions, which are then held together by electrostatic attractions between opposite charges; or by sharing electrons to form covalent bonds. We discussed the nature and characteristics of covalent bonds, noting their relative lengths and energies, as well as polarities. A review of Lewis dot structures and VSEPR theory will prepare you for predicting likely bond arrangements, resonance structures, and molecular geometries. Finally, we compared the relative strengths of the most important intermolecular electrostatic interactions, noting that even the strongest of these—hydrogen bonding—is still much weaker than an actual covalent bond. The next time you’re “browning” some of your food in a pan or the oven, take a moment to consider what’s happening at the atomic and molecular level. It’s not just cooking; it’s science!
Concept Summary
Bonding
· Chemical bonds can be ionic or covalent.
· Elements will form bonds to attain a noble gas-like electron configuration.
· The octet rule states that elements will be most stable with eight valence electrons. However, there are many exceptions to this rule:
o Elements with an incomplete octet are stable with fewer than eight electrons and include H, He, Li, Be, and B.
o Elements with an expanded octet are stable with more than eight electrons and include all elements in period 3 or greater.
o Compounds with an odd number of electrons cannot have eight electrons on each element.
Ionic Bonds
· An ionic bond is formed via the transfer of one or more electrons from an element with a relatively low ionization energy to an element with a relatively high electron affinity.
o Ionic bonds occur between elements with large differences in electronegativity (ΔEN > 1.7), usually between metals and nonmetals.
o A positively charged ion is called a cation. A negatively charged ion is called an anion.
o The resulting electrostatic attraction between the ions causes them to remain in close proximity, forming the bond.
o Ionic compounds form crystalline lattices—large, organized arrays of ions.
· Ionic compounds have unique physical and chemical properties.
o Ionic compounds tend to dissociate in water and other polar solvents.
o Ionic solids tend to have high melting points.
Covalent Bonds
· A covalent bond is formed via the sharing of electrons between two elements of similar electronegativities.
· Bond order refers to whether a covalent bond is a single bond, double bond, or triple bond. As bond order increases, bond strength increases, bond energy increases, and bond length decreases.
· Covalent bonds can be categorized as nonpolar or polar, based on the nature of the elements involved.
o Nonpolar bonds result in molecules in which both atoms have exactly the same electronegativity; some bonds are considered nonpolar when there is a very small difference in electronegativity between the atoms (ΔEN < 0.5), even though they are technically slightly polar.
o Polar bonds form when there is a significant difference in electronegativities (ΔEN = 0.5 to 1.7), but not enough to transfer electrons and form an ionic bond. In a polar bond, the more electronegative element takes on a partial negative charge, and the less electronegative element takes on a partial positive charge.
· Coordinate covalent bonds result when a single atom provides both bonding electrons while the other atom does not contribute any; coordinate covalent bonds are most often found in Lewis acid–base chemistry.
· Lewis dot symbols are a chemical representation of an atom’s valence electrons.
· Drawing a complete Lewis dot structure requires a balance of valence, bonding, and nonbonding electrons in a molecule or ion.
· Formal charges exist when an atom is surrounded by more or fewer valence electrons than it has in its neutral state (assuming equal sharing of electrons in a bond).
· For any molecule with a π (pi) system of electrons, resonance structures exist; these represent all of the possible configurations of electrons—stable and unstable—that contribute to the overall structure.
· The valence shell electron pair repulsion (VSEPR) theory predicts the three-dimensional molecular geometry of covalently bonded molecules. In this theory, electrons—whether bonding or nonbonding—arrange themselves to be as far apart as possible from each other in three-dimensional space, leading to characteristic geometries.
o Nonbonding electrons exert more repulsion than bonding electrons because they reside closer to the nucleus.
o Electronic geometry refers to the position of all electrons in a molecule, whether bonding or nonbonding. Molecular geometry refers to the position of only the bonding pairs of electrons in a molecule.
· The polarity of molecules is dependent on the dipole moment of each bond and the sum of the dipole moments in a molecular structure.
o All polar molecules contain polar bonds.
o Nonpolar molecules may contain nonpolar bonds, or polar bonds with dipole moments that cancel each other.
· σ and π bonds describe the patterns of overlap observed when molecular bonds are formed.
o Sigma (σ) bonds are the result of head-to-head overlap.
o Pi (π) bonds are the result of the overlap of two parallel electron cloud densities.
Intermolecular Forces
· Intermolecular forces are electrostatic attractions between molecules. They are significantly weaker than covalent bonds (which are weaker than ionic bonds).
o London dispersion forces are the weakest interactions, but are present in all atoms and molecules. As the size of the atom or structure increases, so does the corresponding London dispersion force.
o Dipole–dipole interactions, which occur between the oppositely charged ends of polar molecules, are stronger than London forces; these interactions are evident in the solid and liquid phases but negligible in the gas phase due to the distance between particles.
o Hydrogen bonds are a specialized subset of dipole–dipole interactions involved in intra- and intermolecular attraction; hydrogen bonding occurs when hydrogen is bonded to one of three very electronegative atoms—fluorine, oxygen, or nitrogen.
Answers to Concept Checks
· 3.1
1. Ionic bonds form between ions and involve gain or loss of electrons. Covalent bonds occur when electrons are shared between atoms.
2. Any three examples that form incomplete octets (H, He, Li, Be, B) or expanded octets (Period 3 and greater) are acceptable.
3. The polarity in a covalent bond is determined by differences in electronegativity between the two atoms involved.
· 3.2
1. Metals lose electrons because they have low ionization energies, while nonmetals gain electrons because they have high electron affinities. These processes are complementary, leading to the formation of an ionic bond.
2. Some characteristics of ionic compounds include high melting and boiling points due to electrostatic attractions, solubility of ions in water due to interactions with polar solvents, good conductors of heat and electricity, crystal lattice arrangement to minimize repulsive forces, and large electronegativity differences between ions, among other possible answers.
· 3.3
1. Bond strength is defined by the electrostatic attraction between nuclei and electrons; multiple bonds (higher bond order) increases strength. Bond length is a consequence of these attractions. The stronger the bond, the shorter it is. Bond energy is the minimum amount of energy needed to break a bond. The stronger the bond, the higher the bond energy.
2. Nonpolar covalent bonds form with ΔEN = 0 to 0.5. Polar covalent bonds form with ΔEN = 0.5 to 1.7. Ionic bonds form with ΔEN = 1.7 or higher.
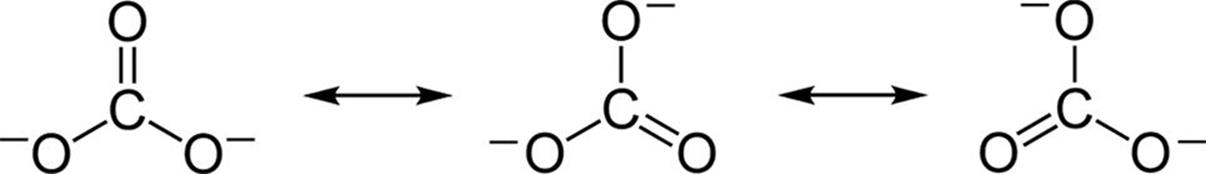
3. 
4. PCl5: trigonal bipyramidal, MgF2: linear, AlF3: trigonal planar, UBr6: octahedral, SiH4: tetrahedral
· 3.4
1. Hydrogen bonding > dipole–dipole interactions > dispersion (London) forces
2. A dipole consists of a segment of a molecule with partial positive and partial negative regions. The positive end of one molecule is attracted to the negative end of another molecule, and vice-versa.
3. To experience hydrogen bonding, a molecule must contain a hydrogen bonded to a very electronegative atom (nitrogen, oxygen, or fluorine).
Equations to Remember
(3.1) Dipole moment: p = qd
(3.2) Formal charge:
![]()
Shared Concepts
· General Chemistry Chapter 1
o Atomic Structure
· General Chemistry Chapter 2
o The Periodic Table
· General Chemistry Chapter 4
o Compounds and Stoichiometry
· Organic Chemistry Chapter 3
o Bonding
· Organic Chemistry Chapter 4
o Analyzing Organic Reactions
· Physics and Math Chapter 5
o Electrostatics and Magnetism