MCAT General Chemistry Review
Chapter 4: Compounds and Stoichiometry
4.2 Representation of Compounds
There are different ways of representing compounds and their constituent atoms. We’ve already reviewed a couple of these systems in Chapter 3 of MCAT General Chemistry Review: Lewis dot structures and VSEPR theory. In organic chemistry, it is common to encounter skeletal representations of compounds, called structural formulas, that show the various bonds between the constituent atoms of a compound. Inorganic (general) chemistry typically represents compounds by showing the constituent atoms without representing the actual bond connectivity or atomic arrangement. For example, the formula C6H12O6 (glucose) tells us that this particular compound consists of six atoms of carbon, twelve atoms of hydrogen, and six atoms of oxygen, but there is no indication of how the different atoms are arranged or how many bonds exist between each of the atoms.
BRIDGE
Many of these representations will be discussed in more detail in Chapter 2 of MCAT Organic Chemistry Review. Understanding the theory behind such representations will help convert between different projections and representations with ease.
LAW OF CONSTANT COMPOSITION
The law of constant composition states that any pure sample of a given compound will contain the same elements in an identical mass ratio. For example, every sample of water will contain two hydrogen atoms for every one oxygen atom, or—in terms of mass—for every one gram of hydrogen, there will be eight grams of oxygen.
REAL WORLD
Even biologically important molecules such as water and amino acids on Earth are, by composition, the same anywhere else in the universe, even though densities and other physical properties may differ.
EMPIRICAL AND MOLECULAR FORMULAS
There are two ways to express the formula of a compound. The empirical formula gives the simplest whole-number ratio of the elements in the compound. The molecular formula gives the exact number of atoms of each element in the compound and is a multiple of the empirical formula. For example, the empirical formula for benzene is CH, while the molecular formula is C6H6. For some compounds, the empirical and molecular formulas are identical, as is the case for H2O. As previously discussed, ionic compounds, such as NaCl or CaCO3, will only have empirical formulas.
BRIDGE
An empirical formula of CH2O is indicative of a monosaccharide. Common monosaccharides include glucose, fructose, and galactose. The structures of these monosaccharides—and of carbohydrates in general—are discussed in Chapter 4 of MCAT Biochemistry Review.
PERCENT COMPOSITION
The percent composition of an element (by mass) is the percent of a specific compound that is made up of a given element. To determine the percent composition of an element in a compound, the following formula is used:
![]()
Equation 4.5
One can calculate the percent composition of an element by using either the empirical or the molecular formula. It is also possible to determine the molecular formula given both the percent compositions and molar mass of a compound. The following examples demonstrate such calculations.
MCAT EXPERTISE
Percent composition is a common way for stoichiometry to be tested on the MCAT. Practice these problems to build up speed and efficiency for Test Day.
Example:
What is the percent composition of chromium in K2Cr2O7?
Solution:
The molar mass of K2Cr2O7 is:
![]()
Calculate the percent composition of Cr:
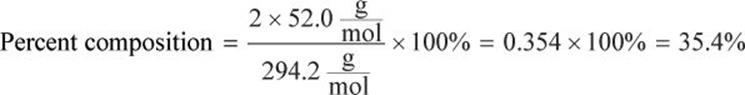
Example:
What are the empirical and molecular formulas of a compound that contains 40.9% carbon, 4.58% hydrogen, and 54.52% oxygen and has a molar mass of ![]()
Method One:
First, determine the number of moles of each element in the compound by assuming a 100-gram sample; this converts the percentage of each element present directly into grams of that element. Then convert grams to moles:
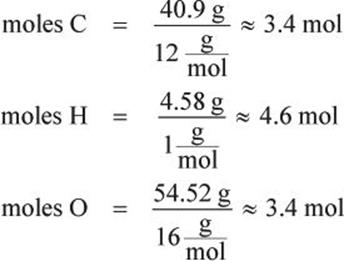
Next, find the simplest whole number ratio of the elements by dividing the number of moles by the smallest number obtained in the previous step.
![]()
Finally, the empirical formula is obtained by converting the numbers obtained into whole numbers by multiplying them by an integer value.
C1H1.33O1 × 3 = C3H4O3
C3H4O3 is the empirical formula. To determine the molecular formula, divide the molar mass by the empirical formula weight. The resulting value gives the number of empirical formula units in the molecular formula.
The formula weight of C3H4O3 is:
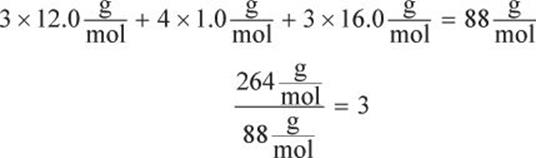
C3H4O3 × 3 = C9H12O9. The molecular formula is C9H12O9.
Method Two:
When the molar mass is given, it is generally easier to find the molecular formula first. This is accomplished by multiplying the molar mass by the given percentages to find the mass of each element present in one mole of compound, then dividing by the respective atomic weights to find the mole ratio of the elements:
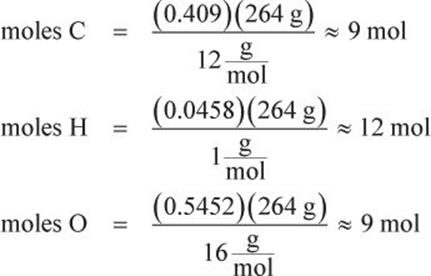
Thus, the molecular formula C9H12O9 is the direct result. The empirical formula can now be found by reducing the subscript ratio to the simplest integer values (C3H4O3).
MCAT EXPERTISE
When there are two methods for approaching a problem, be well-versed in both. Knowing multiple ways to solve a problem will help you tackle questions efficiently.
KEY CONCEPT
The molecular formula is either the same as the empirical formula or a multiple of it. To calculate the molecular formula, you need to know the mole ratio (this will give you the empirical formula) and the molar mass (molar mass divided by empirical formula weight will give the multiplier for the empirical formula-to-molecular formula conversion).
MCAT Concept Check 4.2:
Before you move on, assess your understanding of the material with these questions.
1. What are some similarities and differences between molecular and empirical formulas?
· Similarities:
· Differences:
2. Find the percent composition (by mass) of sodium, carbon, and oxygen in sodium carbonate (Na2CO3):
· Sodium:
· Carbon:
· Oxygen:
3. Experimental data from the combustion of an unknown compound indicates that it is 28.5% iron, 24.0% sulfur, and 49.7% oxygen by mass. What is its empirical formula?