MCAT General Chemistry Review
Chapter 1: Atomic Structure
1.2 Atomic Mass vs. Atomic Weight
There are a few different terms used by chemists to describe the heaviness of an element: atomic mass and mass number, which are essentially synonymous, and atomic weight. While the atomic weight is a constant for a given element and is reported in the Periodic Table, the atomic mass or mass number varies from one isotope to another. In this section, carefully compare and contrast the different definitions of these terms—because they are similar, they can be easy to mix up on the MCAT.
KEY CONCEPT
· Atomic number (Z) = number of protons
· Mass number (A) = number of protons + number of neutrons
· Number of protons = number of electrons (in a neutral atom)
· Electrons are not included in mass calculations because they are much smaller.
ATOMIC MASS
As we’ve seen, the mass of one proton is approximately one amu. The size of the atomic mass unit is defined as exactly ![]() the mass of the carbon-12 atom, approximately 1.66 × 10−24 g. Because the carbon-12 nucleus has six protons and six neutrons, an amu is approximately equal to the mass of a proton or a neutron. The difference in mass between protons and neutrons is extremely small; in fact, it is roughly equal to the mass of an electron.
the mass of the carbon-12 atom, approximately 1.66 × 10−24 g. Because the carbon-12 nucleus has six protons and six neutrons, an amu is approximately equal to the mass of a proton or a neutron. The difference in mass between protons and neutrons is extremely small; in fact, it is roughly equal to the mass of an electron.
The atomic mass of an atom (in amu) is nearly equal to its mass number, the sum of protons and neutrons (in reality, some mass is lost as binding energy, as discussed in Chapter 9 of MCAT Physics and Math Review). Atoms of the same element with varying mass numbers are calledisotopes (from the Greek for “same place”). Isotopes differ in their number of neutrons and are referred to by the name of the element followed by the mass number; for example, carbon-12 or iodine-131. Only the three isotopes of hydrogen, shown in Figure 1.3, are given unique names:protium (Greek: “first”) has one proton and an atomic mass of 1 amu; deuterium (“second”) has one proton and one neutron and an atomic mass of 2 amu; tritium (“third”) has one proton and two neutrons and an atomic mass of 3 amu. Because isotopes have the same number of protons and electrons, they generally exhibit similar chemical properties.
ATOMIC WEIGHT
In nature, almost all elements exist as two or more isotopes, and these isotopes are usually present in the same proportions in any sample of a naturally occurring element. The weighted average of these different isotopes is referred to as the atomic weight and is the number reported on the Periodic Table. For example, chlorine has two main naturally occurring isotopes: chlorine-35 and chlorine-37. Chlorine-35 is about three times more abundant than chlorine-37; therefore, the atomic weight of chlorine is closer to 35 than 37. On the Periodic Table, it is listed as 35.5. Figure 1.4 illustrates the half-lives of the different isotopes of the elements; because half-life corresponds with stability, it also helps determine the relative proportions of these different isotopes.
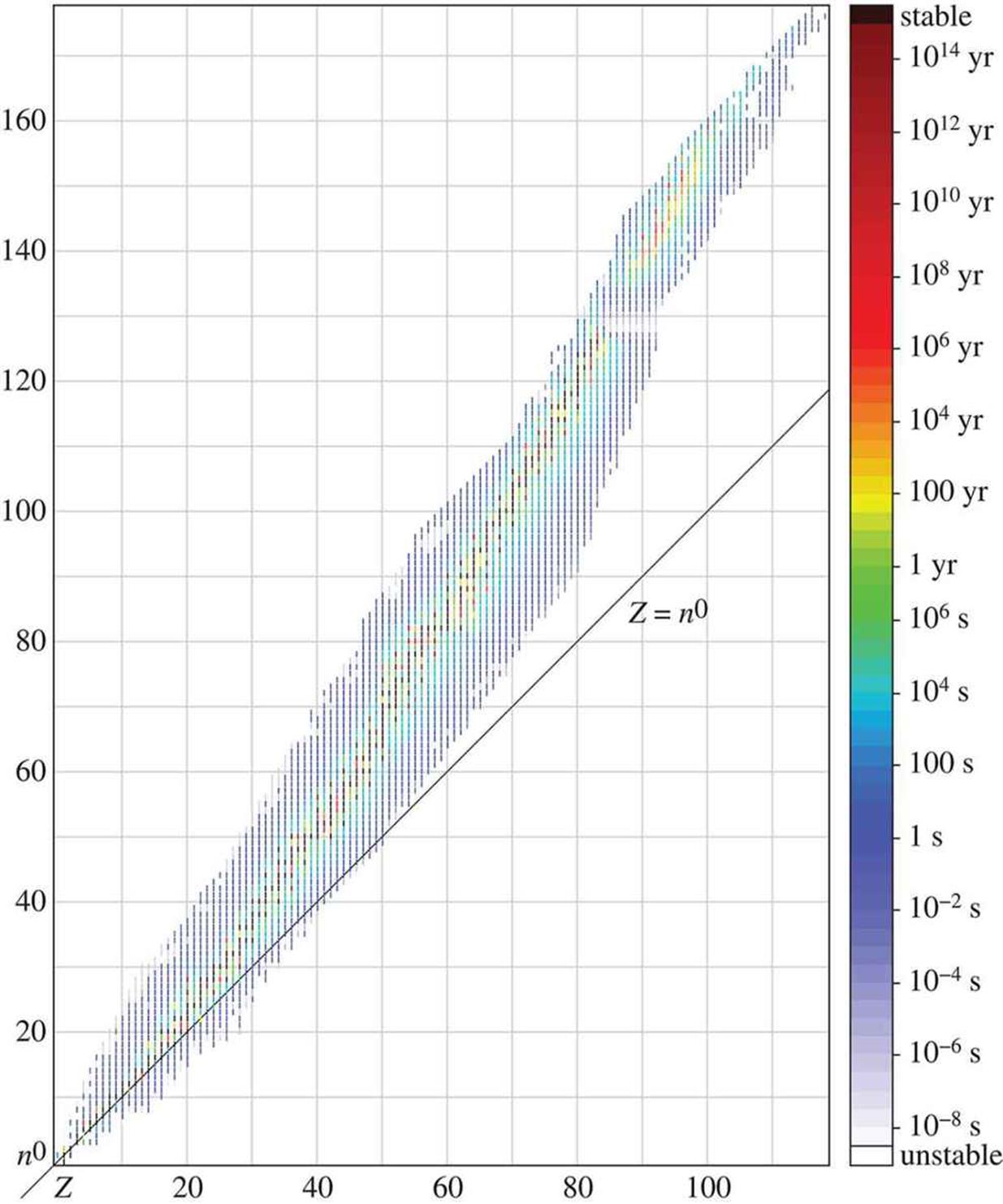 Figure 1.4. Half-Lives of the Different Isotopes of Elements Half-life is a marker of stability; generally, longer-lasting isotopes are more abundant.
Figure 1.4. Half-Lives of the Different Isotopes of Elements Half-life is a marker of stability; generally, longer-lasting isotopes are more abundant.
KEY CONCEPT
When an element has two or more isotopes, no one isotope will have a mass exactly equal to the element’s atomic weight. Bromine, for example, is listed in the Periodic Table as having a mass of 79.9 amu. This is an average of the two naturally occurring isotopes, bromine-79 and bromine-81, which occur in almost equal proportions. There are no bromine atoms with an actual mass of 79.9 amu.
The utility of the atomic weight is that it represents both the mass of the “average” atom of that element, in amu, and the mass of one mole of the element, in grams. A mole is a number of “things” (atoms, ions, molecules) equal to Avogadro’s number, NA = 6.02 × 1023. For example, the atomic weight of carbon is ![]() which means that the average carbon atom has a mass of 12.0 amu (carbon-12 is far more abundant than carbon-13 or carbon-14), and 6.02 × 1023 carbon atoms have a combined mass of 12.0 grams.
which means that the average carbon atom has a mass of 12.0 amu (carbon-12 is far more abundant than carbon-13 or carbon-14), and 6.02 × 1023 carbon atoms have a combined mass of 12.0 grams.
MNEMONIC
Atomic mass is nearly synonymous with mass number. Atomic weight is a weighted average of naturally occurring isotopes of that element.
Example:
Element Q consists of three different isotopes: A, B, andC. Isotope A has an atomic mass of 40 amu and accounts for 60 percent of naturally occurring Q. Isotope B has an atomic mass of 44 amu and accounts for 25 percent of Q. Finally, isotope C has an atomic mass of 41 amu and accounts for 15 percent of Q. What is the atomic weight of element Q?
Solution:
The atomic weight is the weighted average of the naturally occurring isotopes of that element.
0.60 (40 amu) + 0.25 (44 amu) + 0.15 (41 amu) = 24.00 amu + 11.00 amu + 6.15 amu = 41.15 amu.
The atomic weight of element Q is ![]()
MCAT Concept Check 1.2:
Before you move on, assess your understanding of the material with these questions.
1. What are the definitions of atomic mass and atomic weight?
· Atomic mass:
· Atomic weight:
2. While mass is typically written in grams per mole 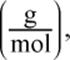 is the ratio moles per gram
is the ratio moles per gram 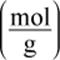 also acceptable?
also acceptable?
3. Calculate and compare the subatomic particles that make up the following atoms.
|
Isotope |
Protons |
Neutrons |
Electrons |
|
19O |
|||
|
16O |
|||
|
17O |
|||
|
19F |
|||
|
16F |
|||
|
238U |
|||
|
240U |