MCAT General Chemistry Review
Chapter 4: Compounds and Stoichiometry
4.6 Ions
Ionic compounds are of particular interest to chemists because certain important types of chemical reactions—acid–base and oxidation–reduction reactions, for instance—commonly take place in ionic solutions. For stoichiometry problems, the goal with ions is to identify oxidation states. This will allow us to determine electron equivalents, balance equations, and deduce chemical formulas from nomenclature.
CATIONS AND ANIONS
In Chapter 3 of MCAT General Chemistry Review, we discussed how ionic compounds are made up of positively charged cations, usually metals, and negatively charged anions, usually nonmetals. This rule does not always hold true for elements like hydrogen, which can act like an anion or cation but is still classified as a nonmetal, as shown in Figure 4.9. Ionic compounds are held together by ionic bonds, which rely on the force of electrostatic attraction between oppositely charged particles.
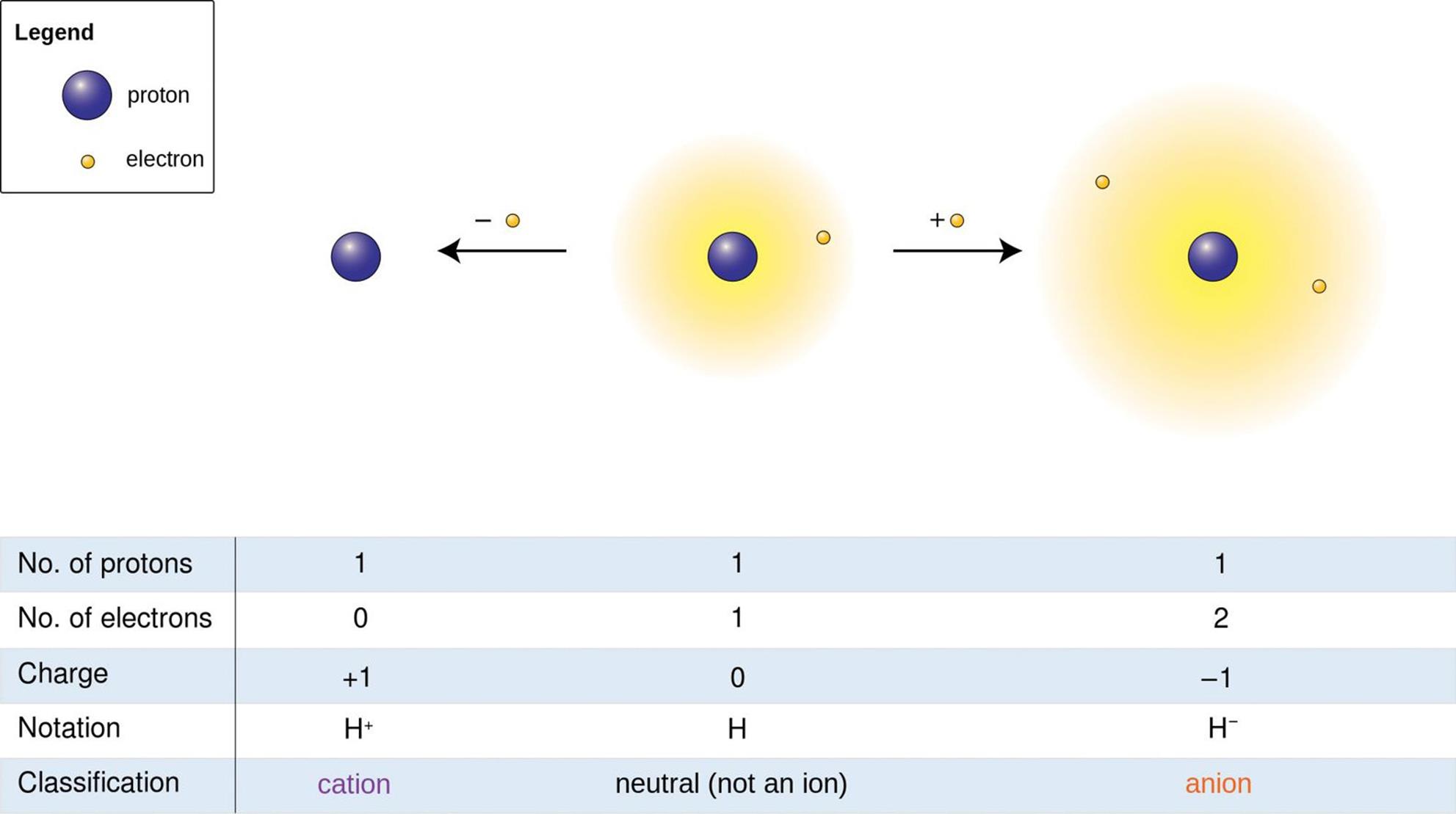 Figure 4.9. Oxidation States of Hydrogen
Figure 4.9. Oxidation States of Hydrogen
BRIDGE
The magnitude of the electrostatic force in an ionic bond follows the same conventions described for Coulomb’s law in Chapter 5 of MCAT Physics and Math Review. The distance between nuclei in ionic bonds is inversely proportional to the force. Therefore, ionic compounds with long bond distances are much more weakly held together.
The nomenclature of ionic compounds is based on the names of the component ions:
1. For elements (usually metals) that can form more than one positive ion, the charge is indicated by a Roman numeral in parentheses following the name of the element.
|
Fe2+ |
Iron(II) |
Cu+ |
Copper(I) |
|
Fe3+ |
Iron(III) |
Cu2+ |
Copper(II) |
2. An older, less commonly used method is to add the endings –ous or –ic to the root of the Latin name of the element to represent the ions with lesser and greater charge, respectively.
|
Fe2+ |
Ferrous |
Cu+ |
Cuprous |
|
Fe3+ |
Ferric |
Cu2+ |
Cupric |
3. Monatomic anions are named by dropping the ending of the name of the element and adding –ide.
|
H− |
Hydride |
S2− |
Sulfide |
|
F− |
Fluoride |
N3− |
Nitride |
|
O2− |
Oxide |
P3− |
Phosphide |
4. Many polyatomic anions contain oxygen and are therefore called oxyanions. When an element forms two oxyanions, the name of the one with less oxygen ends in –ite, and the one with more oxygen ends in –ate.
|
NO2− |
Nitrite |
SO32− |
Sulfite |
|
NO3− |
Nitrate |
SO42− |
Sulfate |
5. In extended series of oxyanions, prefixes are also used. Hypo– and hyper, written as per–, are used to indicate less oxygen and more oxygen, respectively.
|
ClO− |
Hypochlorite |
|
ClO2− |
Chlorite |
|
ClO3− |
Chlorate |
|
ClO4− |
Perchlorate |
6. Polyatomic anions often gain one or more H+ ions to form anions of lower charge. The resulting ions are named by adding the word hydrogen or dihydrogen to the front of the anion’s name. An older method uses the prefix bi– to indicate the addition of a single hydrogen ion.
|
HCO3− |
Hydrogen carbonate or bicarbonate |
|
HSO4− |
Hydrogen sulfate or bisulfate |
|
H2PO4− |
Dihydrogen phosphate |
7. Other common polyatomic ions that may be useful to know are in Table 4.1.
|
Charge |
Formula |
Name |
|
+1 |
NH4+ |
Ammonium |
|
−1 |
C2H3O2− |
Acetate |
|
−2 |
CrO42− |
Chromate |
|
−3 |
BO33− |
Borate |
|
Table 4.1. Other Common Polyatomic Ions |
||
MCAT EXPERTISE
It is unlikely that –ous or –ic endings will be required for most problem-solving. Passages tend to provide reaction schemes that allow you to deduce any unfamiliar compound’s formulas. However, it is still important to understand the nomenclature for discrete questions.
MNEMONIC
The “litest” anions have the fewest oxygens; the heaviest anions ate the most oxygens.
ION CHARGES
Ionic species, by definition, have charge. Cations have positive charge, and anions have negative charge. Some elements are only found naturally in their charged forms, while others may exist naturally in the charged or uncharged state. Some elements can even have several different charges or oxidation states, depending on the other atoms in a compound.
Some of the charged atoms or molecules that are on the MCAT include the active metals—the alkali metals (Group IA or Group 1) and the alkaline earth metals (Group IIA or Group 2), which have charges of +1 and +2, respectively, in the natural state.
BRIDGE
Remember that alkali metals are not typically found in nature in their uncharged state because they are highly reactive with moisture. Instead, they are found as cations in salts (like NaCl).
Nonmetals, which are found on the right side of the Periodic Table, generally form anions. For example, all the halogens (Group VIIA or Group 17) form monatomic anions with a charge of –1 because they already have 7 electrons and aim to fill an octet.
In summary, all elements in a given group tend to form monatomic ions with the same charge (for example, all Group IA elements have a charge of +1 in their ionic state). Note that there are anionic species that contain metallic elements (for example, MnO4− [permanganate] and CrO42−[chromate]); even so, the metals have positive oxidation states. Also note that in the oxyanions of the halogens, such as ClO– and ClO2−, the halogen is assigned a positive oxidation state.
BRIDGE
Oxyanions of transition metals like the MnO4− and CrO42− ions have an inordinately high oxidation number on the metal. As such, they tend to gain electrons in order to reduce this oxidation number and thus make good oxidizing agents. Good oxidizing and reducing agents are discussed in Chapter 4 of MCAT Organic Chemistry Review.
For nonrepresentative elements like many of the transition metals, such as copper, iron, and chromium, there are numerous positively charged states. These states need not be memorized. Experimentally, the color of a solution can be indicative of the oxidation state of a given element in the solution. The same element in different oxidation states can undergo different electron transitions and therefore absorb different frequencies of light. In Figure 4.10, this phenomenon is shown for various plutonium salts with different oxidation states for plutonium indicated in Roman numerals.
 Figure 4.10. Solutions with Various Plutonium Oxidation States
Figure 4.10. Solutions with Various Plutonium Oxidation States
The trends of ionicity, as we’ve described here, are helpful but are complicated by the fact that many elements have intermediate electronegativity and are consequently less likely to form ionic compounds, and by the left-to-right transition from metallic to nonmetallic character on the Periodic Table.
ELECTROLYTES
In spite of the fact that ionic compounds are composed of ions, solid ionic compounds tend to be poor conductors of electricity because the charged particles are rigidly set in place by the lattice arrangement of the crystalline solid. In aqueous solutions, however, the lattice arrangement is disrupted by the ion–dipole interactions between the ionic components and the water molecules. The cations and anions are now free to move, and as a result, the solution of ions is able to conduct electricity.
Solutes that enable solutions to carry currents are called electrolytes. The electrical conductivity of aqueous solutions is governed by the presence and concentration of ions in the solution. Subsequently, the number of electron equivalents being transferred in such a system, such as in electrochemical cells, varies. Pure water, which has no ions other than the very few hydrogen ions and hydroxide ions that result from water’s low-level autodissociation, is a very poor conductor.
MCAT EXPERTISE
Ionic compounds make good electrolytes because they dissolve most readily. Nonpolar covalent compounds are the weakest because they do not form current-carrying ions.
The tendency of an ionic solute to dissolve, or solvate, into its constituent ions in water may be high or low. A solute is considered a strong electrolyte if it dissociates completely into its constituent ions. Examples of strong electrolytes include certain ionic compounds, such as NaCl and KI, and molecular compounds with highly polar covalent bonds that dissociate into ions when dissolved, such as HCl in water. An example of solvation of such compounds is shown in Figure 4.11.
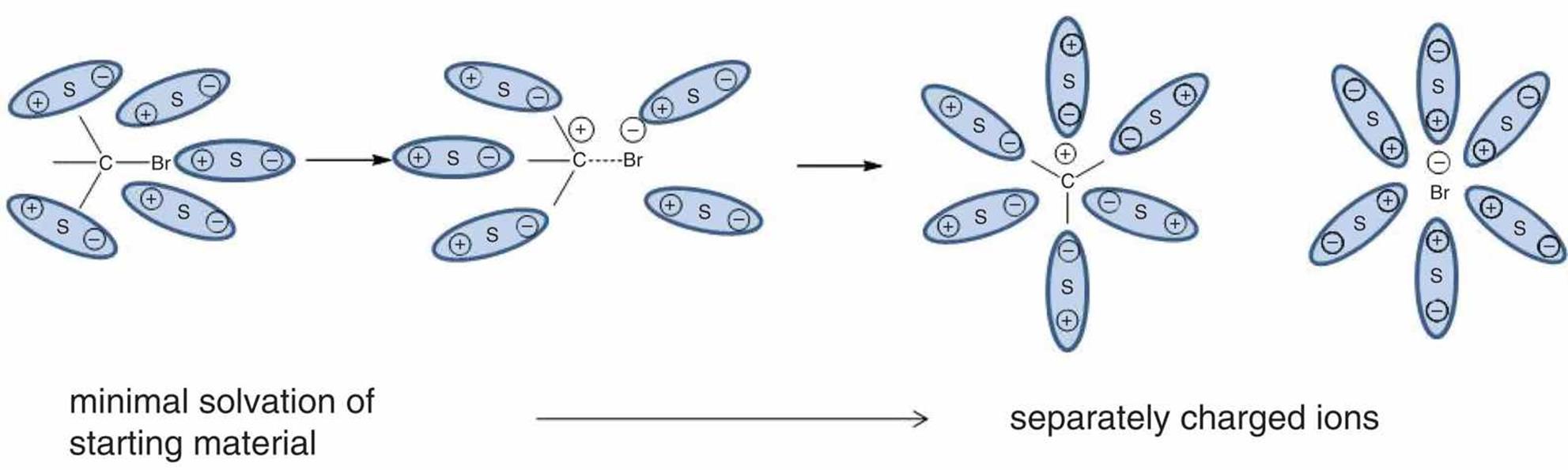 Figure 4.11. Solvation of a Polar Covalent Compound S indicates a solvent particle.
Figure 4.11. Solvation of a Polar Covalent Compound S indicates a solvent particle.
A weak electrolyte, on the other hand, ionizes or hydrolyzes incompletely in aqueous solution, and only some of the solute is dissolved into its ionic constituents. Examples include Hg2I2 (Ksp = 4.5 × 10–29), acetic acid and other weak acids, and ammonia and other weak bases. Many compounds do not ionize at all in aqueous solution, retaining their molecular structure in solution, which may also limit their solubility. These compounds are called nonelectrolytes and include many nonpolar gases and organic compounds, such as O2 (g), CO2 (g), and glucose.
BRIDGE
Because electrolytes ionize in solution, they will produce a larger effect on colligative properties, described in Chapter 8 of MCAT General Chemistry Review, than one would expect from the given concentration.
MCAT Concept Check 4.6:
Before you move on, assess your understanding of the material with these questions.
1. Label the following solutions as electrolytes or nonelectrolytes: (Note: Assume these compounds are all in aqueous solution.)
|
· HCl |
Electrolyte |
Nonelectrolyte |
|
· Sucrose |
Electrolyte |
Nonelectrolyte |
|
· MgBr2 |
Electrolyte |
Nonelectrolyte |
|
· CH4 |
Electrolyte |
Nonelectrolyte |
2. Identify the following ions as cations or anions, and then provide the formula or chemical symbol:
|
Ion |
Cation or Anion |
Formula |
|
Phosphate |
||
|
Hypochlorite |
||
|
Ammonium |
||
|
Phosphide |
||
|
Bicarbonate |
||
|
Nitrite |
||
|
Chromium(II) |