MCAT General Chemistry Review
Chapter 4: Compounds and Stoichiometry
Answers and Explanations
1. DIonic compounds are composed of atoms held together by ionic bonds. Ionic bonds associate charged particles with large differences in electronegativity. Rather than forming molecules or being measured by molecular weight, as in choices (A) and (B), ionic compounds form large arrays of ions in crystalline solids and are measured with formula weights. In ionic bonds, electrons are not really shared but rather are donated from the less electronegative atom to the more electronegative atom, eliminating choice (C).
2. AOf the compounds listed, both choices (B) and (D) are covalent compounds and thus are measured in molecular weights, not formula weights. The formula weight of MgCl2 is much too high (24.3 amu + 2 × 35.5 amu = 95.3 amu per formula unit), eliminating choice (C). Only KCl fits the criteria (39.1 amu + 35.5 amu = 74.6 amu).
3. AFirst, it is helpful to know the molar mass of one mole of H2SO4, which is found by adding the atomic weights of the atoms that constitute the molecule:
![]()
Gram equivalent weight is the weight (in grams) that would release one mole of protons.Because sulfuric acid has two hydrogens per molecule, the gram equivalent weight is 98.1 g divided by 2, or 49.1 g.
4. CThe definition of an empirical formula is a formula that represents a molecule with the simplest ratio, in whole numbers, of the elements comprising the compound. In this case, given the empirical formula CH, any molecule with carbon and hydrogen atoms in a 1:1 ratio would be accurately represented by this empirical formula. Choice (C) is has three carbon atoms and four hydrogen atoms. Both its molecular and empirical formulas would be C3H4 because this formula represents the smallest whole-number ratio of its constituent elements.
5. AThe percent composition by mass of any given element within a molecule is equal to the mass of that element in the molecule divided by the molar mass of the compound, times 100%. In this case, acetone, C3H6O, has
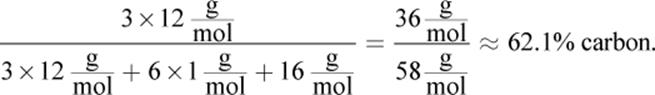
Choice (B), ethanol, is
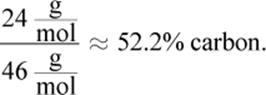
Choice (C), propane, is

Finally, choice (D), methanol, is

6. BThis reaction is a classic example of a neutralization reaction, in which an acid and a base react to form water and a new aqueous compound. Although this reaction also fits the criteria for a double-displacement reaction, choice (C), in which two molecules essentially exchange ions with each other, neutralization is a more specific description of the process.
7. AIn this question, you are first given the masses of both reactants used to start the reaction. To figure out what will be left over, we must first determine which species is the limiting reagent. The formula weight of Na2S is 78.1 grams per formula unit; the formula weight of AgNO3 is 169.9 grams per formula unit. From this, we can determine that we are given 0.5 mol Na2S and 0.67 mol AgNO3. Because we need two moles of AgNO3 for every mole of Na2S, AgNO3 is the limiting reagent, and the correct answer choice will be in grams of Na2S. If 0.67 mol of AgNO3 are used up, and Na2S will be consumed at half the rate of AgNO3 (based on their mole ratio), then 0.33 mol Na2S will be used up. We then have 0.17 mol excess Na2S, which has a mass of 13.0 g.
8. AThis is a question best answered by dimensional analysis. Keeping in mind that molar mass is measured in grams of a substance per moles of that substance, only choice (A) comes out with the units of grams of oxygen. Choice (B) has the units of grams per mole of oxygen, not grams of oxygen. Choice (C) has the units of moles per gram of oxygen. Choice (D) has the units of mol2 per gram of oxygen.
9. DIn the reaction, there is a single displacement, with the silver in silver oxide being replaced by the aluminum to form aluminum oxide. This single-displacement reaction also necessitates a transfer of electrons in an oxidation–reduction reaction; silver, for example, changes from the +2 oxidation state to neutral. Aluminum changes from neutral to the +3 oxidation state.
10.CTypically, both single-displacement and double-displacement reactions have two reactants that swap either one or two components between the two species. Combination reactions, on the other hand, have more reactants than products because the reactants combine together to form the product.
11.BThis description characterizes a combustion reaction because a hydrocarbon acts as a fuel when reacting with oxygen. Carbon dioxide (an oxide) and water are the products of such a reaction.
12.A
The equation given is unbalanced. To start, we must balance the equation:
![]()
The theoretical yield is the amount of product synthesized if the limiting reagent is completely used up. This question therefore asks how much glucose is produced if the limiting reagent is 30 grams of water. We can use the three-fraction method discussed in this chapter to solve for the mass of glucose produced:

Thus, 50 grams of glucose are produced.
13.B
A limiting reagent is by definition a reactant. Because Au and H2S are products, they cannot act as limiting reagents, eliminating choices (C) and (D). Next, realize that what you are shown is an unbalanced equation. To answer this question, we must balance the reaction:
Au2S3 (s) + 3 H2 (g) → 2 Au (s) + 3 H2S (g)
We are given 2 moles of gold(III) sulfide and 5 moles of hydrogen gas. To use up both moles of gold(III) sulfide, we would need 6 moles of hydrogen gas because there is a 1:3 ratio between these reactants. We have only 5 moles of hydrogen gas, so that will have to be the limiting reagent.
14.BThe best electrolytes dissociate readily (have a high dissociation constant) and are ionic compounds with large amounts of cations and anions. This rules out choices (A) and (C). Choice (D) has fewer total ions with a smaller total magnitude of charge and therefore is not as strong an electrolyte as choice (B).
15.CThe simplest approach is to determine the molar mass of the empirical formula. B2H5 has a molar mass of ![]() A molecular formula is simply a multiple of the empirical formula; doubling this quantity will result in the molar mass given in the question stem. Therefore, the compound must be B4H10.
A molecular formula is simply a multiple of the empirical formula; doubling this quantity will result in the molar mass given in the question stem. Therefore, the compound must be B4H10.