MCAT General Chemistry Review
Chapter 5: Chemical Kinetics
Answers and Explanations
1. D
Based on the information given in the question, the rate is first-order with respect to the concentration of the first reactant; when the concentration of that reactant doubles, the rate also doubles. Because the reaction is third-order, the sum of the exponents in the rate law must be equal to 3. Therefore, the reaction order with respect to the other reactant must be 3 – 1 = 2. If the concentration of this second reactant is multiplied by ![]() the rate will be multiplied by
the rate will be multiplied by 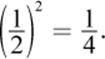
2. D
Before you try to answer this question, you should draw a free energy diagram for the system.
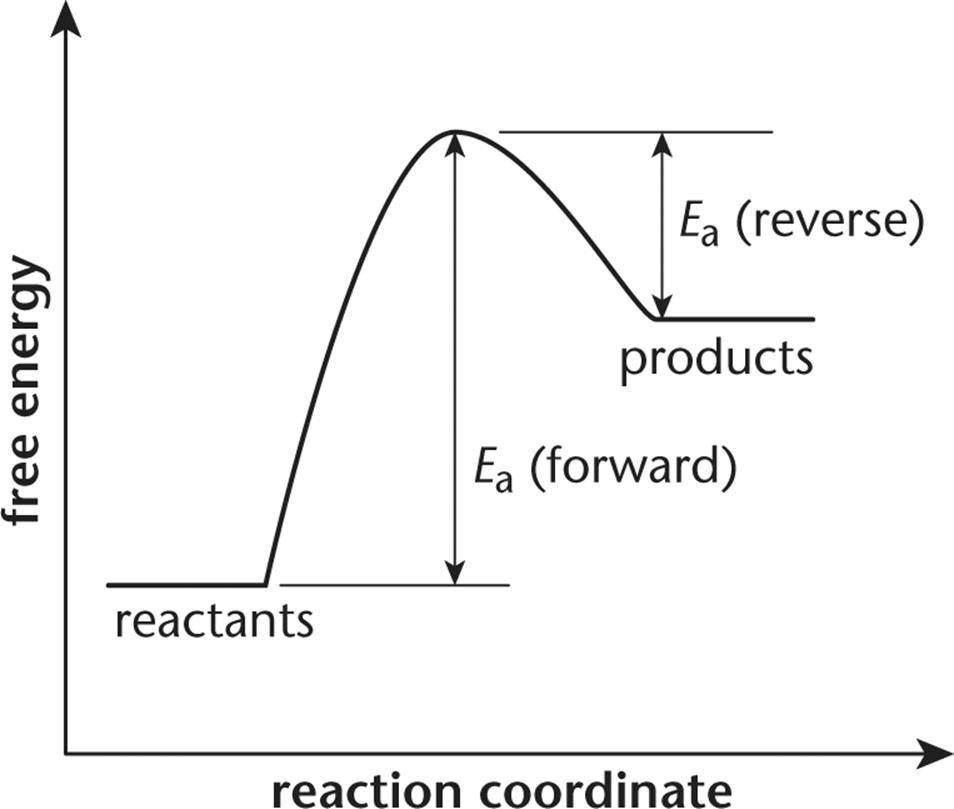
If the activation energy of the forward reaction is greater than the activation energy of the reverse reaction, then the products must have a higher free energy than the reactants. The overall energy of the system is higher at the end than it was in the beginning. The net free energy change is positive, indicating an endergonic (nonspontaneous) reaction. The terms endothermic, choice (A), and exothermic, choice (B), are associated with enthalpy. While free energy does depend on enthalpy, it also depends on entropy; there is not enough information in the question stem to reliably determine the sign of the enthalpy change of the reaction.
3. D
A second-order reaction can be second-order with respect to one reactant, or first-order with respect to two different reactants. In this case, one reactant was increased by a factor of 4. If the reaction is second-order with respect to this reactant, the rate will increase by a factor of 16. If it is first-order with respect to this reactant (and first-order with respect to another reactant), the rate will increase by a factor of 4. We do not know which of these is this correct rate law and, thus, cannot determine the effect on the rate.
4. A
By definition, zero-order reactions are unaffected by the concentrations of any reactants in the reaction. Thus, changing the concentrations of these reactants will not affect the rate.
5. D
The question asks which of the following does NOT affect the rate of the reaction. Temperature directly affects the rate constant (k), making choice (A) incorrect. Changing the partial pressure of a gas will affect the number of effective collisions per time. This makes choice (B)incorrect—but note that concentration changes will not affect the rate of zero-order reactions. Solvents affect the rate of reactions depending on how the reactants interact with the solvent, making choice (C) incorrect. Removing the product of an irreversible reaction, choice (D), should not affect the rate of the reaction because the rate law does not depend on the concentrations of products.
6. D
While increasing the concentration of reactants can alter the reaction rate in first- or higher-order reactions, saturated solutions containing a catalyst have a maximum turnover rate that cannot adjust the rate constant or the reaction rate any higher.
7. A
If the sum of the exponents (orders) of the concentrations of each species in the rate law is equal to 2, then the reaction is second-order. The exponents in the rate law are unrelated to stoichiometric coefficients, so NO2 and Br2 could have any stoichiometric coefficients in the original reaction and still be a second-order reaction, invalidating statement II. Statement III is incorrect because the rate can be affected by a wide variety of compounds. A catalyst, for example, could increase the rate.
8. C
In the first two trials, the concentration of XH4 is held constant, while the concentration of O2 is multiplied by 4. Because the rate of the reaction is also increased by a factor of approximately 4, oxygen must be a first-order reactant. In the last two trials, the concentration of O2 is held constant while the concentration of XH4 is doubled. Because the rate of the reaction is increased by a factor of approximately 4 again, XH4 must be a second-order reactant. Based on this, we can conclude that the experimental rate law is k[XH4]2[O2].
9. B
By definition, a catalyst increases the rate of a reaction by lowering the activation energy, making it easier for both the forward and reverse reactions to overcome this energy barrier. Catalysts are neither used up in the reaction, nor do they alter the equilibrium of a reaction, eliminating choices (A) and (C). Finally, catalysts stabilize the transition state by lowering its energy, not raising it, eliminating choice (D).
10.C
The overall order of a reaction is the sum of the individual orders in the reaction. Therefore, the rate order is 0 + 2 + 1 = 3.
11.D
A system is exergonic if energy is released by the reaction. For exergonic reactions, the net energy change is negative, and the free energy of the final products is lower than the free energy of the initial reactants. Point E, which represents the of the final products, is lower on the energy diagram than point A, which represents the energy of initial reactants. Thus, energy must have been given off, and the reaction is exergonic.
12.B
The activation energy of a reaction is represented by the distance on the y-axis from the energy of the reactants to the peak energy prior to formation of products. The activation energy of the first step of the forward reaction, for example, is equal to the distance along the y-axis from point A to point B. The largest energy increase on this graph occurs during the progress from point E to point D, which represents the first step of the reverse reaction.
13.D
Intermediates exist at “valleys” in reaction diagrams. Reactants, choice (A), are represented by point A. Products, choice (B), are represented by point E. Transition states, choice (C), are represented by points B and D.
14.D
To answer this question, recall that the slow step of a reaction is the rate-determining step. The rate is always related to the concentrations of the reactants in the rate-determining step (not the overall reaction), so NO2 is the only compound that should be included in the correct answer. The concentration of NO2 is squared in the rate law because the stoichiometric coefficient of NO2 in the rate-determining step is 2.
15.D
The faster a reaction can reach its activation energy, the faster it will proceed to completion. Because this question states that all conditions are equal, the reaction with the lowest activation energy will have the fastest rate. In the diagram, choice (D) has the lowest activation energy.