MCAT General Chemistry Review
Chapter 6: Equilibrium
6.3 Kinetic and Thermodynamic Control
Having covered the fundamentals of kinetics and thermodynamics, we come upon a topic that bridges all chemical systems: control of a reaction. In particular, biochemical reactions often require regulation in a precise manner to be useful to an organism. The applications of kinetic and thermodynamic control are common on the MCAT and range from metabolic reactions requiring high-energy phosphate molecules such as ATP to the effects of temperature and solvents on enzyme activity.
The examples below consider unimolecular systems through the lens of the transition state theory. Figure 6.4 shows starting materials (reactants) at a certain energy level. These reactants can undergo two different sets of reactions. At lower temperatures (with smaller heat transfer), a kinetic product is formed. At higher temperatures (with larger heat transfer), a thermodynamic product is formed.
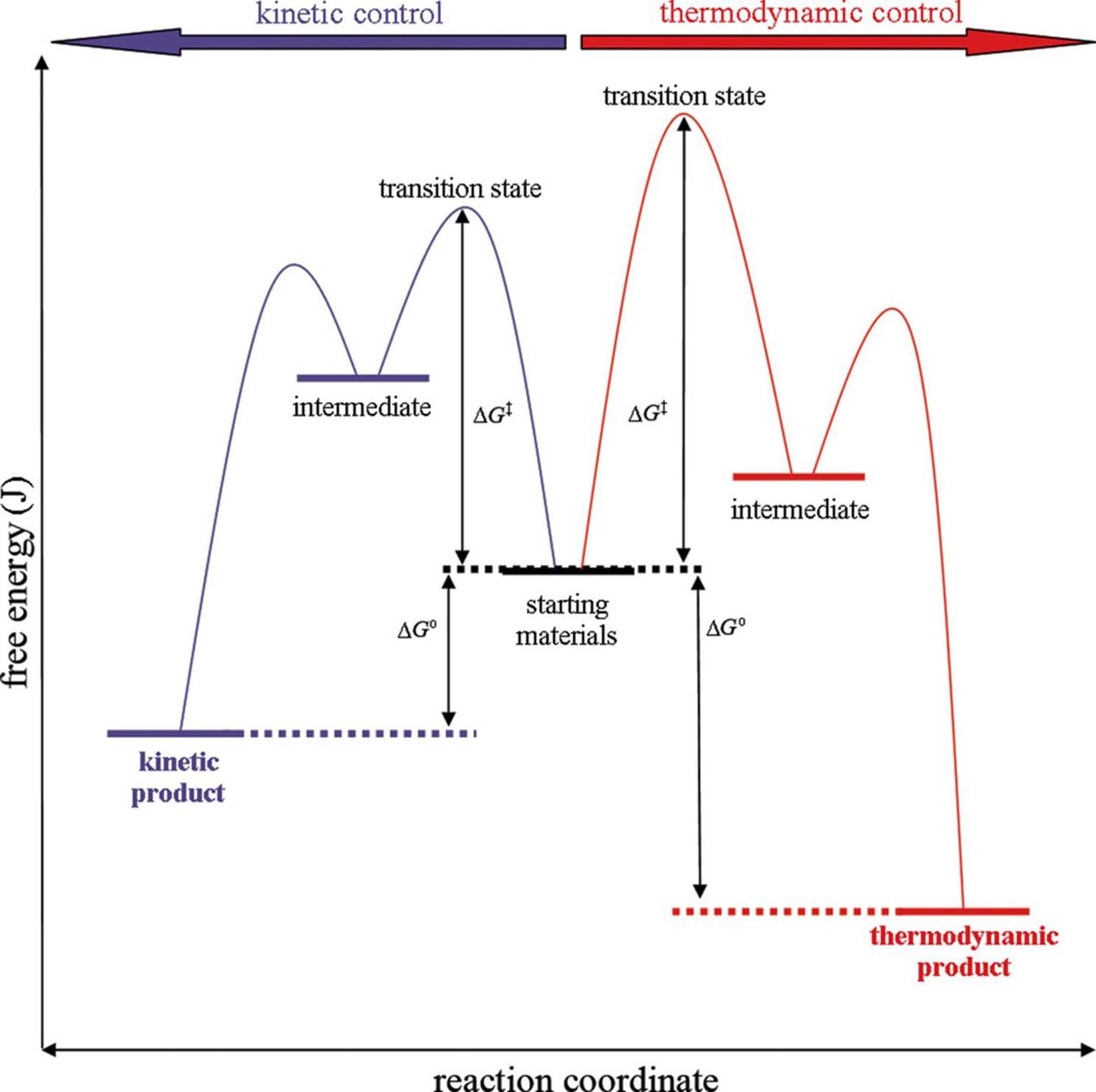 Figure 6.4. Kinetic and Thermodynamic Control of a Reaction The kinetic pathway requires less free energy to reach the transition state, but results in a higher-energy (less stable) product.
Figure 6.4. Kinetic and Thermodynamic Control of a Reaction The kinetic pathway requires less free energy to reach the transition state, but results in a higher-energy (less stable) product.
Note that the free energy that must be added for the kinetic pathway is lower than that of the thermodynamic pathway. Therefore, the kinetic products often form faster than the thermodynamic products and are sometimes called “fast” products. On the other hand, the free energy of the thermodynamic product is significantly lower than that of the kinetic product. Thermodynamic products are therefore associated with greater stability, and with a more negative ΔG than kinetic products.
The stability of organic molecules is covered in Chapter 2 of MCAT Organic Chemistry Review and is dependent on torsional strain, angle strain, and nonbonded strain. In this example, we consider the conversion of 2-methylcyclohexanone to its thermodynamic product and its kinetic product, as shown in Figure 6.5. Both reactions require a base (B–) in order to catalyze the conversion, yet two different products are produced.
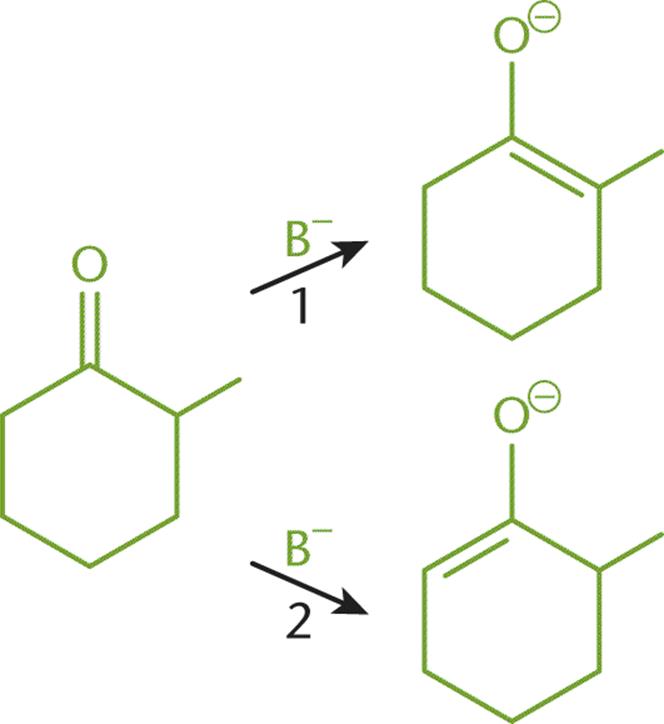 Figure 6.5. Conversion of 2-methylcyclohexanone to (1) Thermodynamic Product and (2) Kinetic Product
Figure 6.5. Conversion of 2-methylcyclohexanone to (1) Thermodynamic Product and (2) Kinetic Product
For the thermodynamic pathway (1), the double bond is located between the carbonyl and methyl groups. It requires more energy to form the transition state of this reaction because the base must overcome the steric hindrance created by the methyl group. The base squeezes in to reach the carbon with the methyl group attached to abstract a proton. However, because the double bond is more substituted than the other pathway, the product of this reaction is more stable and less likely to react further.
For the kinetic pathway (2), the double bond is located between the carbonyl and C-6. This pathway is preferred when there is little heat available because less energy is needed to reach the transition state. The base can more easily reach C-6 to remove a proton, and the resulting enolate can form. This product has a less substituted double bond, which reduces its stability. This lack of stability may leave the ring susceptible to further attack.
MCAT Concept Check 6.3:
Before you move on, assess your understanding of the material with these questions.
1. What conditions favor formation of a kinetic product? A thermodynamic product?
· Kinetic product:
· Thermodynamic product:
2. On a reaction coordinate diagram, how would the kinetic pathway appear as compared to the thermodynamic pathway?